Abstract
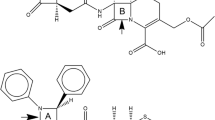
The structures of three β-lactam penem antibiotics—i.e. the sodium[5R-[5α,6α(R*)]]-6-(1-hydroxyethyl)-7-oxo-3-[[(1-pyrrolidinylthioxomethyl)thio]methyl]-4-thia-1-azabicyclo[3.2.0] hept-2-ene-2-carboxylate (compound 1), the [5R-[3(S*),5α,6α(R*)]]-3-[[2-(aminocarbonyl)-1-pyrrolidinyl] methyl]-6-(1-hydroxyethyl)-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (compound 2), and the [5R-[5α,6α(R*)]]-3-[[(2-amino-2-oxoethyl) methylamino]methyl]-6-(1-hydroxyethyl)-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (compound 3)—have been determined by X-ray analyses. In the crystal lattice two conformational isomers of 1 are present, which differ from each other in the spatial arrangement of the dithiocarbamate chain. Compounds 2 and 3 are in zwitterionic form, being the hydrogen of the carboxylic acid moved to the amino nitrogen of the chain at C2. This hydrogen atom, in both molecules, forms intramolecular hydrogen bonds with an oxygen atom of the carboxylate moiety and with the oxygen atom of the amido group of the side chain. The 3D structures of 1, 2, and 3 have been compared with those of previously reported β-lactam penem antibiotics. Particularly, the Woodward parameter and the Cohen distance, which are considered important in determining the antibiotic activity, have been discussed. Least-squares minimizations (RMS) of the distances between nuclei of selected pairs of atoms defining the pharmacological pattern have been performed, comparing five common antibiotics (imipenem, ritipenem, cephaloridine, amoxycillin, and benzylpenicillin) with our compounds. Finally, molecular dynamics calculations have been carried out on the three penem antibiotics at different temperatures. The conformational behavior of the hydroxyethyl chain, the carboxylate group, and the chain at C2 is discussed by considering the variation of some selected dihedral angles.
Similar content being viewed by others
REFERENCES
β-Lactam Antibiotics; Salton, M. R. J., Shockman, G. D. (Eds.); Academic Press: New York, 1981. (b) Chemistry and Biology of β-Lactam Antibiotics; Morin, R. B., Gorman, M. (Eds.); Academic Press: New York, 1982. (c) Knowles, J. Acc. Chem. Res. 1985, 18, 97. (d) Dürckheimer, W.; Blumach, J.; Lattrell, R.; Scheunemann, H. K. Angew. Chem. Int. Ed. Engl. 1985, 24, 180. (e) The Chemistry of β-Lactams; Page, M. I. (Ed.), Blackie Academic and Professional: Glasgow, 1992.
Recent Progress in the Chemical Synthesis of Antibiotics; Lukacs, G., Ohno, M., (Eds.); Springer-Verlag: Berlin, 1990. (b) Sedelmeier, G. Nachr. Chem. Tech. Lab. 1990, 38, 616. (c) Wise, R. Antimicrob. Newsl. 1990, 7, 73. (d) McCombie, S. W.; Ganguly, A. K. Med. Res. Rev. 1988, 8, 393.
Ernest, I.; Gosteli, J.; Greengrass, C. W.; Holick, W.; Jackman, D. E.; Pfaendler, H. R.; Woodward, R. B. J. Am. Chem. Soc. 1978, 100, 8214.
Ratcliffe, R. W.; Albers-Schoenberg, G. The Chemistry of Thienamicin and Other Carbapenem Antibiotics, Gorman, M. (Eds.); Academic Press: New York, 1982 in Reference 1b, 2, 227.
Jefson, M. R.; Hecker, S. J.; Dirlam, J. P. Ann. Rep. Med. Chem. 1994, 29, 113. (b)(Ishiguro, M.; Iwata, H.; Nakatsuka, T.; Tanaka, R.; Maeda, Y.; Noguchi, T. J. Antibiot. 1988, 41, 1685.
Altamura, M.; Giannotti, D.; Perrotta, E.; Sbraci, P.; Pestellini, V.; Arcamone, F.; Satta, G. BioMed. Chem. Lett. 1993, 3, 2159.
Altamura, M.; Perrotta, E.; Sbraci, P.; Pestellini, V.; Arcamone, F.; Cascio, G.; Lorenzi, L.; Satta, G.; Morandotti, G.; Sperning, R. J. Med. Chem. 1995, 38, 4244.
Walker, N.; Stuart, D. D. Acta Crystallogr., Sect. A 1983, 39, 158.
Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. J. Appl. Crystallogr. 1993, 26, 343.
Sheldrich, G. M. SHELX 93, Program for crystal structure determination, Univ. of Göttingen, Germany, 1994.
International Tables for X-ray Crystallography, Vol. 4; Kynoch Press: Birmingham, UK, 1974.
Nardelli, M. Comput. Chem. 1983, 7, 95.
Johnson, C. K. ORTER, Rep. ORNL 3794 Oak Ridge National Laboratory, TN, 1971.
Allen, F. H.; Kennard, O. Cambridge structural database, Chem. Des. Autom. News 1993, 8, 31.
MSI 9685 Scranton Road, San Diego, CA 92121-3752.
Kelly, J. A.; Knox, J. R.; Moews, P. C.; Hite, G. J.; Bartolone, J. B.; Zhao, H.; Joris, B.; Frère J.-M. Ghuysen, J.-M. J. Mol. Biol. 1985, 260, 6449. (b) Kelly, J. A.; Knox, J. R.; Zhao, H.; Frère, J.-M.; Ghuysen, J.-M. J. Mol. Biol. 1989, 209, 281. (c) Knox, J. R.; Moews, P. C. J. Mol. Biol. 1991, 220, 435. (d) Oefner, C.; D'Arcy, A.; Daly, J. J.; Gubernator, K.; Charnas; R. J.; Heinze, I.; Hubschwerien, C.; Winkler, F. K. Nature 1990, 343, 284. (e) Wolff, S. Can. J. Chem. 1994, 72, 1014.
Lamotte, J.; Dive, G.; Ghuysen, J. M. Eur. J. Med. Chem. 1991, 26, 43. (b) Fernandez, B.; Carbaillera, L.; Rios, M. A. Biopolymers 1992, 32, 97. (c) Nangia, A.; Biradha, K.; Desiraju, G. R. J. Chem. Soc. Perkin Trans. 1996, 2, 943. (d) Tanaka, R.; Oyama, Y.; Imajo, S.; Matsuki, S.; Ishiguro, M. Bioorganic Med. Chem. 1997, 5, 1389.
Woodward, R. B. Phil. Trans. R. Soc. Lond. 1980, B289, 239, and references therein.
Cohen, N. C. J. Med. Chem. 1983, 26, 259.
Pfaendler, H. R.; Gosteli, J.; Woodward, R. B.; Rihs, G. J. Am. Chem. Soc. 1981, 103, 4526. (b) Oyama, Y.; Imajo, S.; Tanaka, R.; Ishiguro, M. Acta Crystallogr., Sect. C 1994, 50, 1254. (c) Tanaka, R.; Oyama, Y.; Ishiguro, M. J. Chem. Comm. 1990, 853. (d) Beels, C. M. D.; Abu-Rabie, M. S.; Murray-Rust, P.; Murray-Rust, J. J. Chem. Soc., Chem. Comm. 1979, 665. (e) Capraro, H. G.; Francotte, E.; Kohler, B.; Rihs, G.; Scheider, P.; Scartazzini, R.; Zak, O.; Tosch, W. J. Antibiot. 1988, 41, 759. (f) Iwata, H.; Tanaka, R.; Imajo, S.; Oyama, Y.; Ishiguro, M. J. Chem. Soc. Chem. Comm. 1991, 285. (g) Franceschi, G.; Bedeschi, A.; Rizzo, V.; Vigevani, A.; Oberti, R. Bioorg. Med. Chem. Lett. 1993, 3, 2333.
Ratcliffe, R. W.; Wildonger, K. J.; di Michele, L.; Douglas, A. W.; Hajdu, R.; Goegelman, R. T.; Springer, J. P., Hirshfield, J. J. Org. Chem. 1989, 54, 653.
Sweet, R. M.; Dahl, L. F. J. Am. Chem. Soc. 1970, 92, 5489.
Boles, M. O.; Girven, R. J. Acta Crystallogr., Sect. B 1978, 24, 461.
Csoregh, I.; Palm, T. B. Acta Crystallogr., Sect. B 1977, 33, 2169.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dapporto, P., Paoli, P., Rossi, P. et al. X-ray Structures of Three Penem Antibiotics: Molecular Mechanical and Dynamic Aspects. Structural Chemistry 10, 311–319 (1999). https://doi.org/10.1023/A:1022003303575
Issue Date:
DOI: https://doi.org/10.1023/A:1022003303575