Summary
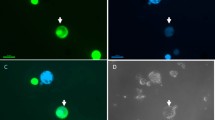
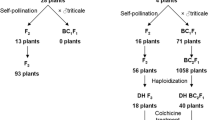
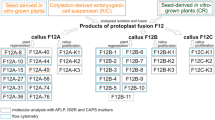
Techniques have been developed for the production of cybrids in Lolium perenne (perennial ryegrass). Gamma-irradiated protoplasts of a cytoplasmically male-sterile breeding line of perennial ryegrass (B200) were fused with iodoacetamide-treated protoplasts of a fertile breeding line (Jon 401). After fusion 25 putative cybrid calli were characterized to determine mitochondrion type and composition of the nuclear genome. Analysis of phosphoglucoisomerase isozyme profiles and determination of the ploidy level by flow cytometry indicated that all of the calli tested essentially contained the nuclear DNA of the fertile line. However, the presence of parts of the nuclear DNA from the sterile line could not be excluded. Southern blotting of total DNA isolated from the parental lines and putative cybrids combined with hybridizations using the mitochondrial probes cox1 and atp6 revealed that the mitochondria of the calli originated from the fertile line (5 calli), the sterile line (5 calli) or from both parental lines (15 calli). The hybridization patterns of the mtDNA from the cybrid calli showed extensive quantitative and qualitative variation, suggesting that fusion-induced inter- or intramolecular mitochondrial recombination had taken place.
Similar content being viewed by others
References
Akagi H, Sakamoto M, Negishi T, Fujimura T (1989) Construction of rice cybrid plants. Mol Gen Genet 215:501–506.
Van Ark HF, Hall RD, Creemers-Molenaar J, Krens FA (1992) High yields of cytoplasts from protoplasts of itLolium perenne and Beta vulgaris using gradient centrifugation. (In press, Plant Cell Tissue and Organ Culture).
Boeshore ML, Lifshitz I, Hanson MR, Izhar S (1983) Novel composition of mitochondrial genomes in Petunia somatic hybrids derived from cytoplasmic male sterile and fertile plants. Mol Gen Genet 190:459–467.
Brears T, Curtis GJ, Lonsdale DM (1989) A specific rearrangement of mitochondrial DNA induced by tissue culture. Theor Appl Genet 77:620–624.
Chowdhury MKU, Schaeffer GW, Smith RL, Debonte LR, Matthews BF (1990) Mitochondrial DNA variation in longterm tissue cultured rice lines. Theor Appl Genet 80:81–87.
Creemers-Molenaar J (1991) Regeneration from protoplasts of perennial ryegrass; progress and applications. In: den Nijs APM, Elgersma A (eds), Fodder crops breeding: achievements, novel strategies and biotechnology. Proc 16th meeting Fodder Crops Section Eucarpia. Wageningen, The Netherlands, pp 123–128.
Creemers-Molenaar J, Van der Valk P, Loeffen JPM, Zaal MACM (1989) Plant regeneration from suspension cultures and protoplasts of Lolium perenne L. Plant Sci 63:167–176.
Creemers-Molenaar J, van Eeuwijk FA, Krens FA (1992) Culture optimization of perennial ryegrass protoplasts. J Plant Physiol 139:303–308.
Dewey RE, Levings CS III, Timothy DH (1985) Nucleotide sequences of atpase subunit 6 of maize mitochondria. Plant Physiol 79:914–919.
Finch RP, Slamet IH, Cocking EC (1990) Production of heterokaryons by the fusion of mesophyll protoplasts of Porteresia coarctata and cell suspension-derived protoplasts of Oryza sativa: a new approach to somatic hybridization in rice. J Plant Physiol 136:592–598.
Gilmour DM, Davey MR, Cocking EC (1989) Production of somatic hybrid tissues following chemical and electrical fusion of protoplasts from albino cell suspensions of itMedicago sativa and M. borealis. Plant Cell Rep 8:29–32.
Glimelius K, Fahlesson J, Landgren M, Sjodin C, Sundberg E (1991) Gene transfer via somatic hybridization in plants. Tib Tech 9:24–30.
Hayashi Y, Kyozuka J, Shimamoto K (1988) Hybrids of rice (Oryza sativa L.) and wild Oryza species obtained by cell fusion. Mol Gen Genet 214:6–10.
Hayward MD, McAdam NJ (1977) Isozyme polymorphism as a measure of distinctiveness and stability in cultivars of Lolium perenne. Z Pflanzenzucht 81:228–234.
Isaac P, Jones VP, Leaver CJ (1985) The maize cytochrome c oxidase subunit I gene: sequence, expression and rearrangement in cytoplasmic male sterile plants. EMBO J 4:1617–1623.
Keller WA, Melchers G (1973) The effect of high pH and calcium on tobacco leaf protoplast fusion. Z Naturforsch 28C: 737–741.
Kemble RJ, Shepard JF (1984) Cytoplasmic DNA variation in a potato protoclonal population. Theor Appl Genet 69:211–216.
Kemble RJ, Barsby TL, Wong RSC, Shepard JF (1986) Mitochondrial DNA rearrangements in somatic hybrids of Solanum tuberosum and Solanum brevidens. Theor Appl Genet 72:787–793.
Kreike CM, de Koning JRA, Krens FA (1990) Non-radioactive detection of single-copy DNA-DNA hybrids. Plant Mol Biol Rep 8:172–179.
Kumar A, Cocking EC (1987) Protoplast fusion: a novel approach to organelle genetics in higher plants. Am J Bot 74:1289–1303.
Kyozuka J, Kaneda T, Shimamoto K (1989) Production of cytoplasmic male sterile rice (Oryza sativa L.) by cell fusion. Bio/Technology 7:1171–1174.
Mettler IJ (1987) A simple and rapid method for minipreparation of DNA from tissue cultured plant cells. Plant Mol Biol 5:346–349.
Östergaard H, Nielsen G, Johansen H (1985) Genetic variation in cultivars of diploid ryegrass, Lolium perenne and L. multiflorum, at five enzyme systems. Theor Appl Genet 69:409–421.
Ozias-Akins P, Ferl RJ, Vasil IK (1986) Somatic hybridization in the gramineae: Pennisetum americanum (L.) K. Schum. (Pearl millet) + Panicum maximum Jacq. (Guinea grass). Mol Gen Genet 203:365–370.
Ozias-Akins P, Tabaeizadeh Z, Pring DR, Vasil IK (1988) Preferential amplification of mitochondrial DNA fragments in somatic hybrids of the Gramineae. Curr Genet 13:241–245.
Rode A, Hartmann C, Falconet D, Lejeune B, Quetier F, Benslimane A, Henry Y, de Buyser J (1987) Extensive mitochondrial DNA variation in somatic tissue cultures initiated from wheat immature embryos. Curr Genet 12:369–376.
Rothenberg M, Boeshore ML, Hanson MR, Izhar S (1985) Intergenomic recombination of mitochondrial genomes in a somatic hybrid plant. Curr Genet 9:615–618.
Saxena PK, King J (1989) Isolation of nuclei and their transplantation into plant protoplasts. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry 9: plant protoplasts and genetic engineering, II. Springer, Berlin Heidelberg New York, pp 328–342.
Sidorov VA, Menczel L, Nagy F, Maliga P (1981) Chloroplast transfer in Nicotiana based on metabolic complementation between irradiated and iodoacetate-treated protoplasts. Planta 152:341–345.
Tabaeizadeh Z, Ferl RJ, Vasil IK (1986) Somatic hybridization in the Gramineae: Saccharum officinarum L. (sugarcane) + Pennisetum americanum (L.) K. Schum. (Pearl millet). Proc Natl Acad Sci USA 83:5616–5619.
Terada R, Kyozuka J, Nishibayashi S, Shimamoto K (1987) Plantlet regeneration from somatic hybrids of rice (Oryza sativa L.) and barnyard grass (Echinochloa oryzicola Vasing). Mol Gen Genet 210:39–43.
Toriyama K, Hinata K (1988) Diploid somatic-hybrid plants regenerated from rice cultivars. Theor Appl Genet 76:665–668.
Vasil V, Ferl RJ, Vasil IK (1988) Somatic hybridization in the Gramineae: Triticum monococcum L. (Einkorn) + Pennisetum americanum (L.) K. Schum. (pearl millet). J Plant Physiol 132:160–163.
Verhoeven HA, Sree Ramulu K, Dijkhuis P (1990) A comparison of the effects of various spindle toxins on metaphase arrest and formation of micronuclei in cell-suspension cultures of Nicotiana plumbaginifolia. Planta 182:408–414.
Wit F (1974) Cytoplasmic male sterility in ryegrasses (Lolium spp.) detected after intergeneric hybridization. Euphytica 23:31–38.
Yang Z-Q, Shikanai T, Yamada Y (1988) Asymmetric hybridization between cytoplasmic male sterile (CMS) and fertile rice (Oryza sativa L.) protoplasts. Theor Appl Genet 76:801–808.
Yang Z-Q, Shikanai T, Mori K, Yamada Y (1989) Plant regeneration from cytoplasmic hybrids of rice (Oryza sativa L.). Theor Appl Genet 77:305–310.
Author information
Authors and Affiliations
Additional information
Communicated by R. Hagemann
Rights and permissions
About this article
Cite this article
Creemers-Molenaar, J., Hall, R.D. & Krens, F.A. Asymmetric protoplast fusion aimed at intraspecific transfer of cytoplasmic male sterility (CMS) in Lolium perenne L.. Theoret. Appl. Genetics 84, 763–770 (1992). https://doi.org/10.1007/BF00224182
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00224182