Summary
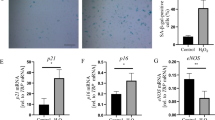
Hypertension, cigarette smoking and diabetes mellitus are well-known risk factors for atherosclerosis and coronary heart disease. Repeated endothelial cell injury and increased lipid entry have been suggested as initiating events in atherogenesis. Our previous studies have demonstrated that the frequency of endothelial cell death and associated endothelial permeability were significantly increased in the aorta of spontaneously hypertensive rats and chronic oral nicotine-treated rats. In the present investigation, we examined the hypothesis that diabetes also increases the frequency of arterial endothelial cell death and hence transendothelial macromolecular transport, which may have some implications in increasing lipid entry and thus accelerating atherogenesis. Diabetes was induced in 15 male Sprague-Dawley rats by intraperitoneal injection of 60 mg streptozotocin per kg body weight. The duration of diabetes was 6 weeks. A group of 15 age-matched rats, injected only with the buffer and maintained over the same time period, served. as the controls. In en face preparations of the thoracic aorta, IgG-containing dead endothelial cells were identified by an indirect immunoperoxidase method, and endothelial leakage to Evans blue-albumin complexes was quantified by fluorescence microscopy. Diabetic rats, compared to control rats, had significantly higher values for the frequency of endothelial cell death (0.77±0.10% vs 0.38±0.04%;p<0.005 by two-tailed, unpaired Student'st-test) and the number density of Evans blue-albumin leaky foci (4.33±0.48/mm2 vs 2.99±0.38/mm2;p<0.05 by two-tailed, unpairedt-test) in the aorta. It is concluded that, similar to the situations in hypertension and nicotine consumption, the observed increase in the frequency of endothelial cell death and macromolecular permeability to large molecules in the aorta in streptozotocin-induced diabetic rats suggest that these changes may contribute to accelerated atherogenesis in diabetes.
Similar content being viewed by others
References
Kannel WB, McGee DL (1979) Diabetes and cardiovascular disease. The Framingham Study. JAMA 241: 2035–2038
Kannel WB, McGee DL (1979) Diabetes and cardiovascular risk factors: the Framingham study. Circulation 59: 8–13
Epstein FH, Ostrander LD, Johnson BC et al. (1965) Epidemiological studies of cardiovascular disease in a total community —Tecumseh, Michigan. Ann Intern Med 62: 1170–1187
Reid DD, Brett GZ, Hamilton PJS, Jarrett RJ, Keen H, Rose G (1974) Cardiorespiratory disease and diabetes among middleaged male civil servants. Lancet I: 469–473
Robertson WB, Strong JP (1968) Atherosclerosis in persons with hypertension and diabetes mellitus. Lab Invest 18: 538–551
Diabetes Drafting Group (1985) Prevalence of small vessel and large vessel disease in diabetic patients from 14 centres. The World Health Organisation Multinational Study of Vascular Disease in Diabetics. Diabetologia 28: 615–640
Ross R (1976) Atherosclerosis: the role of endothelial injury, smooth muscle cell proliferation and platelet factors. Triangle 15: 45–51
Ross R, Glomset JA (1976) The pathogenesis of atherosclerosis. N Engl J Med 295: 369–377
Ross R (1986) The pathogenesis of atherosclerosis-an update. N Engl J Med 314: 488–500
Bierman EL, Brunzell JD (1978) Interrelation of atherosclerosis, abnormal lipid metabolism and diabetes mellitus. In: Katzen HM, Mahler RJ (eds) Advances in Modern Nutrition, vol. 2. Hemisphere Publishing Corporation, Washington London, pp 187–210
Dolgov VV, Zaikina OE, Bondarenko MF, Repin VS (1982) Aortic endothelium of alloxan diabetic rabbits: a quantitative study using scanning electron microscopy. Diabetologia 22: 338–343
Christiansen JS, Gammelgaard J, Frandsen M, Parving HH (1981) Increased kidney size, glomerular filtration rate and renal plasma flow in short-term insulin-dependent diabetes. Diabetologia 20: 451–456
Hostetter TH, Troy JL, Brenner BM (1981) Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int 19: 410–415
Woods LL, Mizelle HL, Hall JE (1987) Control of renal hemodynamics in hyperglycemia: possible role of tubuloglomerular feedback. Am J Physiol 252: F65-F73
Pugliese G, Tilton RG, Williamson JR (1991) Glucose-induced metabolic imbalances in the pathogenesis of diabetic vascular disease. Diabetes Metab Rev 7: 35–59
Parving HH (1976) Increased microvascular permeability to plasma proteins in short- and long-term juvenile diabetes. Diabetes 25[Suppl 2]: 884–889
Tucker BJ (1990) Early onset of increased transcapillary albumin escape in awake diabetic rats. Diabetes 39: 919–923
Williamson JR, Chang K, Tilton RG et al. (1987) Increased vascular permeability in spontaneously diabetic BB/W rats and in rats with mild versus severe streptozotocin-induced diabetes: prevention by aldose reductase inhibitors and castration. Diabetes 36: 813–821
Kilzer P, Chang K, Marvel J et al. (1985) Albumin permeation of new vessels is increased in diabetic rats. Diabetes 34: 333–336
Pugliese G, Tilton RG, Speedy A et al. (1989) Effects of very mild versus overt diabetes on vascular haemodynamics and barrier function in rats. Diabetologia 32: 845–857
Arbogast BW, Berry DL, Newell CL (1984) Injury of arterial endothelial cells in diabetic, sucrose-fed and aged rats. Atherosclerosis 51: 31–45
Wickline KM, Fischer VW (1985) Incorporation of [3H]thymidine into myocardial capillary cells in streptozotocin-diabetic rats. Exp Mol Pathol 43: 135–141
Naeser P (1986) Cell replication in retinal vessels of streptozotocin diabetic mice. Acta Ophthalmol 64: 439–440
Lorenzi M, Cagliero E, Toledo S (1985) Glucose toxicity for human endothelial cells in culture: delayed replication, disturbed cell cycle, and accelerated death. Diabetes 34: 621–627
Stout RW (1982) Glucose inhibits replication of cultured human endothelial cells. Diabetologia 23: 436–439
Lorenzi M, Montisano DF, Toledo S, Barrieux A (1986) High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest 77: 322–325
Lorenzi M, Cagliero E (1991) Perspectives in diabetes: pathobiology of endothelial and other vascular cells in diabetes mellitus. Call for data. Diabetes 40: 653–659
Lin SJ, Jan KM, Chien S (1990) Role of dying endothelial cells in transendothelial macromolecular transport. Arteriosclerosis 10: 703–709
Lin SJ (1989) Effects of cell turnover and leaky junctions on arterial macromolecular permeability — relation to atherogenesis. [Ph.D. dissertation] Columbia University, New York
Harris KH, MacLeod KM (1988) Influence of the endothelium on contractile responses of arteries from diabetic rats. Eur J Pharmacol 153: 55–64
Lin SJ, Jan KM, Chien S (1990) Temporal and spatial changes in macromolecular uptake in rat thoracic aorta and relation to [3H]thymidine uptake. Atherosclerosis 85: 229–238
Hansson GK, Schwartz SM (1983) Evidence for cell death in vascular endothelium in vivo and in vitro. Am J Pathol 112: 278–286
Grahan RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14: 291–302
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD (1989) Cell growth and division. In: Robertson M (ed) Molecular Biology of The Cell, 2nd edn. Garland Press, New York London, pp 762–768
Snedecor GW, Cochran WG (1980) Statistical methods, 7th edn. The Iowa State University Press, Iowa
Lin SJ, Jan KM, Schuessler G, Weinbaum S, Chien S (1988) Enhanced macromolecular permeability of aortic endothelial cells in association with mitosis. Atherosclerosis 73: 223–232
Marshall M (1980) Experimental and clinical results concerning regression of atherosclerosis. Folia Angiol 28: 80–84
Vracko R, Benditt EP (1974) Manifestations of diabetes mellitus. Their possible relationship to an underlying cell defect. Am J Pathol 75: 204–224
Stetz EM, Majno G, Joris I (1979) Cellular pathology of the rat aorta. Pseudovascuoles and mio-endothelial herniae. Virchows Arch [A] 383: 135–148
Weinbaum S, Tzeghai G, Ganatos P, Pfeffer R, Chien S (1985) Effects of cell turnover and leaky junctions on arterial macromolecular transport. Am J Physiol 248: H945-H960
Lin SJ, Jan KM, Weinbaum S, Chien S (1989) Transendothelial transport of low density lipoprotein in association with cell mitosis in rat aorta. Arteriosclerosis 9: 230–236
Rakieten N, Rakieten ML, Nadkarni MV (1963) Studies of the diabetogenic action of streptozotocin. Cancer Chemother Rep 29: 91–98
Arison RN, Ciaccio EI, Glitzer MS, Cassaro JA, Pruss MP (1967) Light and electron microscopy of lesions in rats rendered diabetic with streptozotocin. Diabetes 16: 51–56
Bhuyan BK (1970) The action of streptozotocin on mammalian cells. Cancer Res 30: 2017–2023
Dawber TR, Kannel WB, Revotskie N, Stokes J, Kagan A, Gordon T (1959) Some factors associated with the development of coronary heart disease: six years' follow-up experience in the Framingham study. Am J Pub Health 49: 1349–1356
The pooling project research group (1978) Relationship of blood pressure, serum cholesterol, smoking habit, relative weight and ECG abnormalities to incidence of major coronary events: final report of the pooling project. J Chron Dis 31: 201–306
Wu CH, Chi JC, Jerng JS, Lin SJ, Jan KM, Wang DL, Chien S (1990) Transendothelial macromolecular transport in the aorta of spontaneously hypertensive rats. Hypertension 16: 154–161
Lin SJ, Hong CY, Chang MS, Chiang BN, Chien S (1992) Chronic nicotine exposure increases aortic endothelial cell death and enhances transendothelial macromolecular transport in rats. Arterioscler Thromb 12: 1305–1312
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lin, S.J., Hong, C.Y., Chang, M.S. et al. Increased aortic endothelial cell death and enhanced transendothelial macromolecular transport in streptozotocin-diabetic rats. Diabetologia 36, 926–930 (1993). https://doi.org/10.1007/BF02374474
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02374474