Abstract
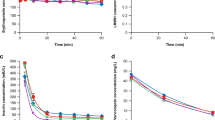
Despite the improvements in the development of dialyzer membranes with greater hemocompatibility, an activation of the coagulation system occurs when blood comes into contact with exogenous surfaces. The large number of heparin dosage regimens demonstrate the difficulty to adapt general therapeutic guidelines. Low molecular weight heparin (Fragmin®) was administered as a single bolus dose for anticoagulation during 58 acute dialyses. Anti-Xa-activity, the plasma levels of the lysosomal elastase of the polymorphnuclear granulocytes (“PMN-elastase”) and of the thrombin-antithrombin III-complex (TAT) were measured at hourly intervals. Therapeutic anti-Xa-levels did not show evidence of sufficient inhibition of thrombin formation. The PMN-elastase increased by 180 ng/ml 3 h after administration of the bolus dose, with no further increase occurring (plateau phase). This was considered to reflect adequate anticoagulative activity. Where anticoagulation was inadequate, the elastase values rose consistently. After 2 h the increase of the PMN-elastase showed that — and to what extent — coagulation had been activated. The determination of PMN-elastase, using the IMAC-principle, is a method which can be performed quickly with any conventional autoanalyzer. It makes it possible to monitor adequate anticoagulation, but PMN-elastase results must be proven during routine use before recommendation as a routine test.
Similar content being viewed by others
References
Hörl WH, Steinhauer HB, Schollmeyer P (1985) Plasma levels of granulocyte elastase during hemodialysis: effect of different dialyzer membranes. Kidney Int 85:791–796
Hörl WH, Steinhauer HB, Riegel W, Schollmeyer P, Schäfer RM, Heidland A (1988) Effect of different dialyzer membranes on plasma levels of granulocyte elastase. Kidney Int 33:90–91
Heimark RL, Kurachi K, Davie EW (1980) Surface activation of blood coagulation, fibrinolysis and kinin formation. Nature 286:456–560
Chenoweth DE, Henderson LW (1987) Complement activation during hemodialysis: laboratory evaluation of hemodialyzers. Artif Org 11:155–162
Müller-Berghaus G, Hoege R, Heinrich D, Brand RR, Berger MS, Meissel R, Hehrlein FW (1984) Granulocyte elastase release and changes of coagulation parameters during and after extracorporal circulation. Thromb Haemost 54:200–201
Pelzer H, Fuhge P, Lange H, Heimburger N (1982) Interactions between dialysis membranes and the coagulation system during hemodialysis. Life support systems. Proc IX Ann meeting ESAO 1982, pp 16–19
Lane DA, Ireland H, Flynn A, Anastassiades E, Curtis JR (1986) Hemodialysis with LMW heparin: dosage requirements for the elimination of extracorporeal fibrin formation. Nephrol Dial Transplant I:179–187
Feinstein DI (1982) Diagnosis and management of disseminated intravascular coagulation: the role of heparin therapy. Blood 60:284–287
Hörl WH, Jochum M, Neumann S, Heidland A (1985) Freisetzung von Granulozyten-Elastase bei akutem Nierenversagen und während der Hämodialysebehandlung bei chrenisch niereninsuffizienten Patienten. In: Neue Wege in der Entzürdungsdiagnostik: PMN-Elastase. GIT, Darmstadt, pp 43–52
Hörl WH, Jochum M, Heidland A, Fritz H (1983) Release of granulocyte proteinase during hemodialysis. Am J Nephrol 3:213–217
Jochum M, Fritz H (1985) Granulozytän Elastase als Marker der unspezifischen Proteolyse entzündlicher Erkrankungen. In: Neue Wege in der Entzündungsdiagnostik: PMN Elastase, GIT, Darmstadt, pp 1–8
Lang H, Dreher M, Heubner A (1981) Diagnostische Validität der Plasma-Elastase als prädiktiver, biochemischer Marker für infektiöse bzw. entzündliche Komplikationen. Dtsch Ges Klin Chem e.V. — Mitteilungen 1/89:10–16
Schäfer RM, Herfs N, Ormanns W, Hoerl WH, Heidland A (1988) Change of elastase and cathepsin G content in polymorphnuclear leucocytes. Clin Nephrol 29:307–311
Haas S, Haas P, Blümel G (1986) Niedermolekulare Heparine. Eine Übersicht über Wirkprofil und bisherige klinische Anwendung. Hämostasiologie 6:180–182
Aiach M, Dreyfus G, Michaud A, Relland J, Murawski M, Leclerc M, Carpentier A (1984) Low molecular weight (LMW) heparin derivatives in experimental extra-corporal circulation (EC). Haemostasis 14:325–323
Henny CP, Ten Cate JW, van Bronswijk H, Ten Cate H, Surachno S, Wilmik JM, Ockelford PA (1983) Use of a new heparinoid as anticoagulant during acute hemodialysis of patients with bleeding complications. Lancet 28:890–893
Ljundberg B, Blombäck M, Johnsson H, Lins LE (1987) A single dose of a low molecular weight heparin fragment for anticoagulation during hemodialysis. Clin Nephrol 27:31–35
Schrader J, Valentin R, Tönnis HJ, Hildebrand U, Stibbe W, Armstrong VW, Kandt M, Köstering H, Wuellhorst E (1985) Low molecular weight heparin in hemodialysis and hemofiltration patients. Kidney Int 28:823–829
Seifert R, Borchert W, Letendre P, Knutsch P, Knutson R, Cipolle R (1986) Heparin kinetics during hemodialysis: Variation in sensitivity, distribution volume, and dosage. Ther Drug Monit 8:32–36
Pelzer H, Schwarz A, Heimburger N (1988) Determination of human thrombin-antithrombin III complex in plasma with a enzyme-linked immunosorbent assay. Thromb Haemost 59:101–106
Fish WW, Björk J (1979) Release of a two-chain form of antithrombin from antithrombin-thrombin complex. Eur J Biochem 101:31–38
Owen WG (1975) Evidence for the formation of an ester between thrombin and heparin cofactor. Biochim Biophys Acta 405:380–387
Fritz H, Jochum M (1986) Lysosomal and systemspecific proteinases as inflammatory mediators. Fresenius Z Anal Chem 324:226
Egbring R, Seitz R, Wolf M, Lerch I, Havemann K (1987) Plasma derivate replacement in disseminated intravascular coagulation (DIC) induced by septic disorders with highly elevated elastase alpha-1-PI-complex. 2nd Int Congr Proteases. May 1987
Stötzer KE, Amiral J, Spanuth E (1988) Neue Methoden zur Bestimmung von Fibrinspaltprodukten (D-Dimere). Lab Med 12:51–59
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Swars, H., Hafner, G., Weilemann, L.S. et al. Acute dialysis: PMN-elastase as a new parameter for controlling individual anticoagulation with low molecular weight heparin (Fragmin®). Intensive Care Med 17, 52–56 (1991). https://doi.org/10.1007/BF01708410
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01708410