Abstract
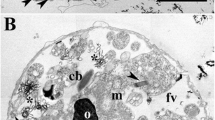
The morphological and ultrastructural characteristics of the cyanobacterium Mastigocladus laminosus growing under N2-fixing conditions were examined with light and electron microscopy. Vegetative cells in narrow filaments contained randomly arranged segments of thylakoid membrane, centrally located carboxysomes (polyhedral bodies), peripherally located lipid bodies, and large numbers of polysaccharide granules in addition to nuclear material and ribosomes. The ultrastructural characteristics of cells in wide filaments were similar, except for increased numbers of carboxysomes and lipid bodies. Heterocytes and proheterocysts developed at a variety of locations in narrow filaments, wide filaments, and the lateral branches off of wide filaments. Akinetes were not observed in any of the filaments. The morphological characteristics of heterocysts and proheterocysts were variable and depended on those of the vegative cells from which the heterocysts and proheterocysts developed. Mature M. laminosus heterocysts were somewhat similar to those formed in other cyanobacterial genera, but they possessed a number of distinct and unique ultrastructural characteristics, including (i) the absence of a fibrous and, possibly, a laminated wall layer, (ii) the presence many closely packed membranes throughout most of the cytoplasm, and (iii) the presence of unidentified, spherical inclusion bodies of variable electron density.
Similar content being viewed by others
References
Adams DG, Carr NG (1981) The developmental biology of heterocyst and akinete formation in cyanobacteria. CRC Crit Rev Microbiol 9:45–100
Allen MM, Smith AJ (1969) Nitrogen chlorosis in blue-green algae. Arch Microbiol 69:114–120
Balkwill DL, Maratea D, Blakemore RP (1980) Ultrastructure of a magnetotactic spirillum. J Bacteriol 141:1399–1408
Boussiba S, Richmond AE (1980) C-phycocyanin as a storage protein in the blue-green alga Spirulina platensis. Arch Microbiol 125:143–147
Bradley S, Carr NG (1976) heterocyst and nitrogenase development in Anabaena cylindrica. J Gen Microbiol 96:175–184
Daley RJ, Brown SR (1973) Chlorophyll, nitrogen, and photosynthetic patterns during growth and senescence of two blue-green algae. J Phycol 9:395–401
Giesy RM (1964) A light and electron microscope study of interlamellar polyglucoside bodies in Oscillatoria chalybia. Amer J Bot 51:388–396
Jensen TE (1968) Electron microscopy of polyphosphate bodies in a blue-green alga, Nostoc pruniforme. Arch Mikrobiol 62:144–152
Kulasooriya SA, Lang NJ, Fay P (1972) The heterocysts of blue-green algae. III. Differentiation and nitrogenase activity. Proc Roy Soc Lond B 181:199–209
Lang NJ (1965) Electron microscopic study of heterocyst development in Anabaena azollae Strasburger. J Phycol 1:127–134
Lang NJ, Fay P (1971) The heterocysts of blue-green algae. II. Details of ultrastructure. Proc Roy Soc Lond B 178:193–203
Lau RH, MacKenzie MM, Doolittle WF (1977) Phycocyanin synthesis and degradation in the blue-green bacterium Anacystis nidulans. J Bacteriol 132:771–778
Marcenko E (1961) Licht- und elektronenmikroskopische Untersuchungen an der Thermalalge Mastiglocladus laminosus Cohn. Acta Bot Croatica 20/21:47–74
Martin TC, Wyatt JT (1974) Comparative physiology and morphology of six strains of stigonematacean blue-green algae. J Phycol 10:57–65
Nierzwicki SA, Maratea D, Balkwill DL, Hardie LP, Mehta VB, Stevens, SE, Jr (1982) Ultrastructure of the cyanobacterium, Mastigocladus laminosus. Arch Microbiol 133:11–19
Ownby JD, Shannahan M, Hood EH (1979) Protein synthesis and degradation in Anabeana during nitrogen starvation. J Gen Microbiol 110:255–261
Pankratz HS, Bowen CC (1963) Cytology of blue-green algae. I. The cells of Symploca muscorum. Amer J Bot 50:387–399
Peat A, Whitton BA (1967) Environmental effects on the structure of the bluegreen alga, Chlorogloea fritschii. Arch Mikrobiol 57:155–180
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Schwabe GH (1960) Über den thermobionten Kosmopoliten Mastigocladus laminosus Cohn. Hydrol 22:759–792
Shively JM (1974) Inclusion bodies of prokaryotes. Ann Rev Microbiol 28:167–187
Spurr AR (1969) A low-viscosity epoxy embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Stevens SE, Jr, Balkwill DL, Paone DAM (1981a) The effects of nitrogen limitation on the ultrastructure of the cyanobacterium Agmenellum quadruplicatum. Arch Microbiol 130:204–212
Stevens SE, Jr, Paone DAM, Balkwill DL (1981b) Accumulation of cyanophycin granules as a result of phosphate limitation in Agmenellum quadruplicatum. Plant Physiol 67:716–719
Stevens SE, Jr, Patterson COP, Myers J (1973) The production of hydrogen peroxide by blue-green algae: a survery. J Phycol 9:427–430
Thurston EL, Ingram LO (1971) Morphology and fine structure of Fischerella ambigua. J Phycol 7:203–210
Vasconcelos de L, Fay P (1974) Nitrogen metabolism and ultrastructure in Anabaena cylindrica. I. The effect of nitrogen starvation. Arch Microbiol 96:271–279
Wilcox M, Mitchison GJ, Smith RJ (1973) Pattern formation in the blue-green alga Anabaena. II. Controlled proheterocyst regression. J Cell Sci 13:637–649
Wolk CP (1973) Physiology and cytological chemistry of blue-green algae. Bacteriol Rev 37:21–101
Wood NB, Haselkorn R (1980) Control of phycobiliprotein proteolysis and heterocyst differentiation in Anabaena. J Bacteriol 141:1375–1385
Yamanaka G, Glazer AN (1980) Dynamic aspects of phycobilisome structure. Phycobilisome turnover during nitrogen starvation in Synechococcus sp. Arch Microbiol 124:39–47
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nierzwicki-Bauer, S.A., Balkwill, D.L. & Stevens, S.E. Morphology and ultrastructure of the cyanobacterium Mastigocladus laminosus growing under nitrogen-fixing conditions. Arch Microbiol 137, 97–103 (1984). https://doi.org/10.1007/BF00414447
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00414447