Abstract
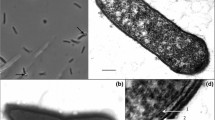
An extremely thermophilic anaerobic fermentative eubacterium growing at temperatures between 50 and 80°C (opt.: 65°C) was isolated from an Icelandic hot spring. The cells were Gram-negative motile rods, about 1.8 μm in length, and 0.6 μm in width occurring singly and in pairs. About 50% of the cells formed large spheroids at one end similar to Fervidobacterium nodosum. The new isolate H 21 differed from Fervidobacterium nodosum by a 6 mol % higher GC-content of its DNA (41 mol %), its ability to grow on cellulose, and insignificant DNA homology. The lipids of isolate H 21 were similar to that of members of “Thermotogales”. 16S rRNA sequencing of isolate H 21 and Fervidobacterium nodosum indicated (a) that isolate H 21 represents a new species of the genus Fervidobacterium which we name Fervidobacterium islandicum and (b) that the genus Fervidobacterium belongs to the “Thermotogales” branch.
Similar content being viewed by others
References
Allen MB (1959) Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol 32:270–277
Balch WE, Wolfe RS (1976) New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminatium in a pressurized atmosphere. Appl Environ Microbiol 32:781–791
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: Reevaluation of a unique biological group. Microbiol Rev 43:260–296
Bergmeyer HU (1974) Methoden der enzymatischen Analyse. Verlag Chemie, Weinheim
Birnstiel ML, Sells BH, Purdom IF (1972) Kinetic complexity of RNA molecules. J Mol Biol 63:21–39
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. J Biochem Physiol 37:911–917
De Rosa M, Gambacorta A, Huber R, Lanzotti V, Nicolaus B, Stetter KO, Trincone A (1988) Lipid structures in Thermotoga maritima. J Chem Soc Chem Commun 1300–1301
Engel M (1989) Charakterisierung des Hauptproteins der äußeren Zellhülle von Thermotoga maritima und verwandter Arten. Diploma Thesis, Ludwig-Maximilian University of Munich, Federal Republic of Germany
Gillespie S, Gillespie D (1971) Ribonucleic acid — deoxyribonucleic acid hybridization in aqueous solutions and in solutions containing formamide. Biochem J 125:481–487
Gutell RR, Woese CR (1990) Higher order structural elements in ribosomal RNAs: Pseudoknots and the use of noncanonical pairs. Proc Natl Acad Sci USA 87 (in press)
Hilpert R, Winter J, Hommes W, Kandler O (1981) The sensitivity of archaebacteria to antibiotics. Zbl Bakt Hyg I Abt Orig C2:11–20
Huber R, Langworthy TA, König H, Thomm M, Woese CR, Sleytr UB, Stetter KO (1986) Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90°C. Arch Microbiol 144:324–333
Huber R, Woese CR, Langworthy TA, Fricke H, Stetter KO (1989) Thermosipho africanus gen. nov., represents a new genus of thermophilic eubacteria within the “Thermotogales”. System Appl Microbiol 12:32–37
Jannasch HW, Huber R, Belkin S, Stetter KO (1988) Thermotoga neapolitana sp. nov of the extremely thermophilic, eubacterial genus Thermotoga. Arch Microbiol 150:103–104
Kelly RB, Cozzarelli NR, Deutscher MP, Lehman JR, Kornberg A (1970) Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem 245:39–45
König H (1984) Isolation and characterization of Methanobacterium uliginosum sp. nov. from a marshy soil. Can J Micriobiol 30:1477–1481
Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR (1985) Rapid determination of the 16S ribosomal RNA sequences for phylogenetic analysis. Proc Natl Acad Sci USA 82:6955–6959
Langworthy TA (1982) Lipids of Thermoplasma In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 88. Academic Press, New York, pp 396–406
Langworthy TA (1985) Lipids of archaebacteria. In: Woese CR, Wolfe RW (eds) The bacteria, vol VIII. Academic Press, Orlando, pp 459–497
Langworthy TA, Pond IL (1986) Membranes and lipids of thermophiles. In: Brock TD (ed) Thermophiles: general, molecular, and applied microbiology. John Wiley and Sons, New York, pp 107–135
Langworthy TA, Holzner G, Zeikus JG, Tornabene TG (1983) Iso-and anteiso-branched glycerol diethers of the thermophilic anaerobe Thermodesulfotobacterium commune. System Appl Microbiol 4:1–17
Lauerer G, Kristjansson JK, Langworthy TA, König H, Stetter KO (1986) Methanothermus sociabilis sp. nov., a second species within the Methanothermaceae growing at 97°C. System Appl Microbiol 8:100–105
Marmur JA (1961) A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol 3:208–218
Marmur J, Doty P (1961) Thermal renaturation of deoxyribonucleic acids. J Mol Biol 3:585–594
Marmur J, Doty P (1962) Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
Ouchterlony Ö (1962) Diffusion-in-gel methods for immunological analysis II. Progr Allergy 6:30–154
Oyaizu H, Debrunner-Vossbrinck B, Mandelco L, Studier JA, Woese CR (1986) The green non-sulfur bacteria: a deep branching in the eubacterial line of descent. System Appl Microbiol 9:47–53
Patel BKC, Morgan HW, Daniel RM (1985) Fervidobacterium nodosum gen. nov. and spec. nov., a new chemoorganotrophic, caldoactive, anaerobic bacterium. Arch Microbiol 141:63–69
Rachel R, Wildhaber I, Stetter KO, Baumeister W (1988) The structure of the surface protein of Thermotoga maritima. In: Sleytr UB, Messner P, Pum D, Sara M (eds) Proceedings of the second international workshop on S-layers in procaryotes. Springer, Berlin Heidelberg New York, pp 83–86
Rhuland LE, Work E, Denman RF, Hoare DS (1955) The behaviour of the isomers of α,ε-diaminopimelic acid on paper chromatograms. J Am Chem Soc 77:4844–4846
Schleifer KH, Kandler O (1972) Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36:407–477
Stetter KO (1982) Ultrathin mycelia-forming organisms from submarine volcanic areas having an optimum growth temperature of 105°C. Nature 300:258–260
Weisburg WG, Tully JG, Rose DL, Petzel JP, Oyaizu H, Yang D, Mandelco L, Sechrest J, Lawrence TG, van Etten J, Maniloff J, Woese CR (1989) A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol 171:6455–6467
Windberger E, Huber R, Trincone A, Fricke H, Stetter KO (1989) Thermotoga thermarum sp. nov. and Thermotoga neopolitana occurring in African continental solfataric springs. Arch Microbiol 151:506–512
Woese CR (1987) Bacterial evolution. Microbiol Rev 51:221–271
Woese CR, Gutell RR (1989) Evidence for several higher order structural elements in ribosomal RNA. Proc Natl Acad Sci USA 86:3119–3122
Woese CR, Sogin M, Stahl DA, Lewis BJ, Bonen L (1976) A comparison of the 16S ribosomal RNAs from mesophilic and thermophilic bacilli. J Mol Evol 7:197–213
Zillig W, Stetter KO, Wunderl S, Schulz W, Priess H, Scholz I (1980) The Sulfolobus-“Caldariella”-group: Taxonomy on the basis of the structure of DNA-dependent RNA polymerases. Arch Microbiol 125:259–269
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huber, R., Woese, C.R., Langworthy, T.A. et al. Fervidobacterium islandicum sp. nov., a new extremely thermophilic eubacterium belonging to the “Thermotogales”. Arch. Microbiol. 154, 105–111 (1990). https://doi.org/10.1007/BF00423318
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00423318