Abstract
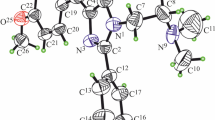
HLö 7 dimethanesulfonate (1-[[[4-(aminocarbonyl)pyridinio] methoxy] methyl] -2,4-bis [(hydroxyimino) methyl]pyridinium dimethanesulfonate) is a broad-spectrum reactivator against highly toxic organophosphorus compounds. The compound was synthesized by a new route with the carcinogenic bis(chloromethyl)ether being substituted by the non-mutagenic bis(methylsulfonoxymethyl)ether. The very soluble dimethanesulfonate of obidoxime was also prepared by this way. HLö 7 dimethanesulfonate is the first water-soluble salt of HLö 7 that should be suitable for the wet/dry autoinjector technology, because aqueous solutions of HLö 7 are not very stable (calculated shelf-life 0.2 years when stored at 8°C, 1 M solution, pH 2.5). The crystalline preparation contains 96% of thesyn/syn-isomer, less than 2% of thesyn/anti-isomer and some minor identified by-products. HLö 7 was very efficient in reactivating acetylcholinesterase (AChE) blocked by organophosphates as long as ageing did not prevent dephosphylation. HLö 7 was superior to HI 6 (1-[[[4-(aminocarbonyl)pyridinio]methoxy]methyl]-2-[(hydroxyimino)methyl]pyridinium dichloride) in reactivating soman and sarin-inhibited AChE from erythrocytes, and literature data indicate that HLö 7 exceeds HI 6 by far in reactivating tabun-inhibited AChE. In atropine-protected, soman-poisoned mice HLö 7 was three times more potent than HI 6 (protective ratio 5 versus 2.5), and in sarin-poisoned mice HLö 7 was 10 times more potent than HI 6 (protective ratio 8 for both oximes). In atropine-protected guinea-pigs HLö 7 was less effective than HI 6 (protective ratio: 2.3 versus 5.2 for soman; 5.2 versus 6.8 for sarin; 4.3 versus 3.8 for tabun). The mean survival time of anaesthetized guinea-pigs exposed to 5 LD50 soman (6.3 min) was increased by atropine (27 min) and atropine + HLö 7 (57 min). HLö 7 alone did not prolong the survival. The most impressive effect of HLö 7 was on respiration: 3 min after i.v. injection of HLö 7 and atropine, the depressed respiration increased rapidly to 60% of control and remained at that level during the observation period (60 min). With atropine alone, respiration recovered only slowly. Behavioural and physiologic parameters were determined in atropine-protected mice exposed to a sublethal soman dose. The running performance was significantly improved by HLö 7. Even central symptoms, e.g. hypothermia and convulsions, were decreased markedly by HLö 7 (evaluation 60 min after poisoning). The pharmacokinetic data for HLö 7 in male beagle dogs are similar to those of HI 6. After i.v. injection: t1/2α = 5 min; t1/2ß = 46 min; VD = 0.24 1/kg; Clp1 = 3.7 ml x min−1 x kg−1; Clren= 3.2 ml x min−1 x kg−1; renal excretion of unchanged HLö 7 = 86%. After i. m. injection: t1/2abs = 14 min; t1/2ß = 48 min; Vd = 0.27 1/kg; Clp1= 3.9 ml x min−1 x kg−1; Clren= 2.7 ml x min−1 x kg−1; renal excretion of unchanged HLö 7 = 76%; bioavailability >95%. Plasma protein binding was <5%; HLö 7 did not permeate into red cells. A dose of 20 μmol/kg was well tolerated both after i.v. and i.m. administration. In anaesthetized dogs (chloralose) HLö 7 i.v. (20 (imol/kg) showed marginal hypotensive effects, whereas 50 μmol/kg resulted in decreased mean blood pressure (−15%) and blood flow (−30%) without reflex tachycardia. One out of four dogs developed a circulatory shock syndrome with anuria. Respiration varied only transiently. Blood gases and pH were not influenced. Similar cardiovascular effects were observed in anaesthetized (urethane) guinea-pigs. In isolated guinea-pig hearts (Langendorff) sinus and ventricular heart rate were not influenced by HLö 7 <500 μM. HLö 7 antagonized both carbachol and nicotine effects. Red cell AChE was inhibited by HLö 7 by up to 50%; C50 about 100 μM. Previously, HLö 7 was shown to block ganglionic transmission (IC50= 500 μM), probably due to ion-channel blockade. These data indicate that HLö 7 combines ganglion blocking, anticholinergic and indirect cholinergic properties like other bispyridinium compounds. The results suggest that HLö 7 may be tolerated by man at a dose of 10 μmol/kg. Vital functions are not expected to be impaired. At such a dose (250–500 mg), which can be injected by an autoinjector, HLö 7 is expected to be superior to HI 6.
Similar content being viewed by others
References
Adolph EF (1949) Quantitative relations in the physiological constitutions of mammals. Science 109: 579–585
Alberts P (1990) A new H-oxime restores rat diaphragm contractility after esterase inhibition in vitro. Eur J Pharmacol 184: 191–194
Alkondon M, Rao KS, Albuquerque EX (1988) Acetylcholinesterase reactivators modify the functional properties of the nicotinic acetylcholine receptor ion channel. J Pharmacol Exp Ther 245: 543–556
Benschop HP, Keijer JH (1966) On the mechanism of ageing of phosphonylated cholinesterases. Biochim Biophys Acta 128: 586–588
Bisa K, Fischer G, Müller O, Oldiges H, Zoche E (1964) Die Antidotwirkung von Bis-[4-hydroxyiminomethyl-pyridinium-(1)-methyl]-äther-dichlorid bei mit Alkylphosphat vergifteten Ratten. Arzneimittelforschung 14: 85–88
Boskovic B, Kovacevic V, Jovanovic D (1984) PAM-2 Cl, HI 6 and HGG 12 in soman and tabun poisoning. Fundam Appl Toxicol 4: S106-S115
Burness DM, Wright CJ, Perkins WC (1977) Bis(methylsulfonoxymethyl)-ether. J Org Chem 42: 2910–2913
Cetkovic S, Cvetkovic M, Jandric D, Cosic M, Boskovic B (1984) Effect of 2-PAM Cl, HI 6, and HGG 12 in poisoning by tabun and its thiocholine-like analog in the rat. Fundam Appl Toxicol 4: S116-S123
Clement JG (1981) Toxicology and pharmacology ofbis-pyridinium oximes. Insight into the mechanism of action vs soman poisoning in vivo. Fundam Appl Toxicol 1: 193–202
Clement JG (1982) HI 6: Reactivation of central and peripheral acetylcholinesterase following inhibition by soman, sarin and tabun in vivo in the rat. Biochem Pharmacol 31: 1283–1287
Clement JG (1983) Efficacy ofmono-, andbis-pyridinium oximes versus soman and tabun poisoning in mice. Fundam Appl Toxicol 3: 533–535
Clement JG (1984) Role of aliesterase in organophosphate poisoning. Fundam Appl Toxicol 4: S96-S105
Clement JG (1991 a) Variability of sarin-induced hypothermia in mice: investigation into incidence and mechanism. Biochem Pharmacol 42: 1316–1318
Clement J (1991 b) Central actions of acetylcholinesterase oxime reactivators. Fundam Appl Toxicol (submitted)
Clement JG, Lockwood PA (1982) HI 6, an oxime which is an effective antidote of soman poisoning: a structure-activity study. Toxicol Appl Pharmacol 64: 140–146
Clement JG, Hansen AS, Boulet CA (1992) Efficacy of HLö 7 and pyrimidoxime as antidotes of nerve agent poisoning in mice. Arch Toxicol 66: 216–219
DFG Deutsche Forschungsgemeinschaft (1987) Maximum concentrations at the workplace and biological tolerance values for working material. VCH Verlagsgesellschaft mbH, Weinheim, p 57
van Dongen CJ, Elskamp RM, de Jong LPA (1987) Influence of atropine upon reactivation and ageing of rat and human erythrocyte acetylcholinesterase inhibited by soman. Biochem Pharmacol 36: 1167–1169
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7: 88–95
Endres W, Spuhler A, ten Bruggencate G (1989) Acetylcholinesterase reactivators antagonize epileptiform bursting induced by paraoxon in guinea-pig hippocampal slices. J Pharmacol Exp Ther 251: 1181–1186
Engelhard H, Erdmann WD (1963) Ein neuer Reaktivator für durch Alkylphosphat gehemmte Acetylcholinesterase Klin Wochenschr 41: 225–227
Engelhard N, Erdmann WD (1964) Beziehungen zwischen chemischer Struktur und Cholinesterase reaktivierender Wirksamkeit bei einer Reihe neuer bis-quartärer Pyridin-4-aldoxime. Arzneimittelforschung 14: 870–875
Erdmann WD, Engelhard H (1964) Pharmakologisch-toxikologische Untersuchungen mit dem Dichlorid des Bis-[4-hydroxyiminomethyl-pyridinium-(1)-methyl]-äthers, einem neuen Esterase-Reaktivator. Arzneimittelforschung 14: 5–11
Eyer P, Lierheimer E, Schneller M (1984) Reactions of nitrosochloramphenicol in blood. Biochem Pharmacol 33: 2299–2308
Eyer P, Hell W, Kawan A, Klehr H (1986) Studies on the decomposition of the oxime HI 6 in aqueous solution. Arch Toxicol 59: 266–271
Eyer P, Hagedorn I, Ladstetter B (1988) Study on the stability of the oxime HI 6 in aqueous solution. Arch Toxicol 62: 224–226
Eyer P, Ladstetter B, Schäfer W, Sonnenbichler J (1989) Studies on the stability and decomposition of the Hagedorn-oxime HLö 7 in aqueous solution. Arch Toxicol 63: 59–67
Fonnum F, Sterri SH (1981) Factors modifying the toxicity of organophosphorus compounds including soman and sarin. Fundam Appl Toxicol 1: 143–147
Fonnum F, Sterri SH, Aas P, Johnsen H (1985) Carboxylesterases, importance for detoxification of organophosphorus anticholinesterases and trichothecenes. Fundam Appl Toxicol 5: S29-S38
Gaustad R, Johnsen H, Fonnum F (1991) Carboxylesterases in guineapig. A comparison of the different isoenzymes with regard to inhibition by organophosphorus compounds in vivo and in vitro. Biochem Pharmacol 42: 1335–1343
Glick D (1937) Properties of choline esterase in human serum. Biochem J 31: 521–525
Gramstad T, Haszeldine RN (1956) Perfluoroalkyl derivatives of sulphur. Part IV. Perfluoroalkanesulphonic acids. J Chem Soc 173–180
Hackley BE, Steinberg GM, Lamb JC (1959) Formation of potent inhibitors of AChE by reaction of pyridinaldoximes with isopropyl methylphosphonofluoridate (GB). Arch Biochem Biophys 80: 211–214
Hagedorn I, Gündel WH, Schoene K (1969) Reaktivierung phosphorylierter Acetylcholinesterase mit Oximen: Beitrag zum Studium des Reaktionsablaufes. Arzneimittelforschung 19: 603–606
Hagedorn I, Stark I, Lorenz P (1972) Reaktivierung phosphorylierter Acetylcholinesterase — Abhängigkeit von der Aktivator-Acidität. Angew Chem 84: 354–356
Hagedorn I, Stark I, Schoene K, Schenkel H (1978) Reaktivierung phosphorylierter Acetylcholinesterase. Isomere bisquartäre Salze von Pyridinaldoximen. Arzneimittelforschung 28: 2055–2057
Hamilton MG, Lundy PM (1989) HI 6 therapy of soman and tabun poisoning in primates and rodents. Arch Toxicol 63: 144–149
Heilbronn E, Tolagen B (1965) Toxogonin in sarin, soman and tabun poisoning. Biochem Pharmacol 14: 73–77
van Helden HPM, de Lange J, Busker RW, Melchers BPC (1991) Therapy of organophosphate poisoning in the rat by direct effects of oximes unrelated to ChE reactivation. Arch Toxicol 65: 586–593
Irwin S (1968) Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioural and physiologic state of the mouse. Psychopharmacology 13: 222–257
de Jong LPA, Ceulen DI (1978) Anticholinesterase activity and rate of decomposition of some phosphylated oximes. Biochem Pharmacol 27: 857–863
de Jong LPA, Wolring GZ (1980) Reactivation of acetylcholinesterase inhibited by 1,2,2′-trimethylpropyl methylphosphonofluoridate (soman) with HI 6 and related oximes. Biochem Pharmacol 29: 2379–2387
de Jong LPA, Wolring GZ (1984) Stereospecific reactivation by some Hagedorn-oximes of acetylcholinesterases from various species including man, inhibited by soman. Biochem Pharmacol 33: 1119–1125
de Jong LPA, Wolring GZ (1985) Aging and stereospecific reactivation of mouse erythrocyte and brain acetylcholinesterase inhibited by soman. Biochem Pharmacol 34: 142–145
de Jong LPA, Verhagen MAA, Langenberg JP, Hagedorn I, Löffler M (1989) The bispyridinium-dioxime HLö 7. A potent reactivator for acetylcholinesterase inhibited by the stereoisomers of tabun and soman. Biochem Pharmacol 38: 633–640
Josselson J, Sidell FR (1978) Effects of intravenous thiamine on pralidoxime kinetics. Clin Pharmacol Ther 24: 95–100
Karger MH, Mazur Y (1971) Mixed sulfonic-carboxylic anhydrides. I. Synthesis and thermal stability. New syntheses of sulfonic anhydrides. J Org Chem 36: 528–531
Kirsch DM, Weger N (1981) Effects of the bispyridinium compounds HGG 12, HGG 42, and obidoxime on synaptic transmission and NAD(P)H-fluorescence in the superior cervical ganglion of the rat in vitro. Arch Toxicol 47: 217–232
Klimmek R, Eyer P (1985) Pharmacokinetics and toxicity of the oxime HGG 12 in dogs. Arch Toxicol 57: 237–242
Klimmek R, Eyer P (1986) Pharmacokinetics and pharmacodynamics of the oxime HI 6 in dogs. Arch Toxicol 59: 272–278
Kusic R, Boskovic B, Vojvodic V, Jovanovic D (1985) HI 6 in man: blood levels, urinary excretion, and tolerance after intramuscular administration of the oxime to healthy volunteers. Fundam Appl Toxicol 5: S89-S97
Kusic R, Jovanovic D, Randjelovic S, Joksovic D, Todorovic V, Boskovic B, Jokanovic M, Vojvodic V (1991) HI 6 in man: Efficacy of the oxime in poisoning by organophosphorus insecticides. Hum Exp Toxicol 10: 113–118
Ladstetter B (1990) Stabilität und metabolisches Schicksal neuer Antidote gegen Organophosphate. Thesis, Univ. München
Ligtenstein DA, Kossen SP (1983) Kinetic profile in blood and brain of the cholinesterase reactivating oxime HI 6 after intravenous administration to the rat. Toxicol Appl Pharmacol 71: 177–183
Litchfield JT, Wilcoxon F (1949) A simplified method of evaluating dose-effect experiments. Pharmacol Exp Ther 96: 99–113
Löffler M (1986) Quartäre Salze von Pyridin-2,4-dialdoxim als Gegenmittel für Organophosphat-Vergiftungen. Thesis, Univ. Freiburg
Lorenz HP (1974) Syn- und anti-Aldoxime N-heteroaromatischer Aldoxime: Darstellung, Ermittlung ihrer Konfiguration und Stabilität sowie Studium der Isomerisierungsreaktion. Thesis, Univ. Freiburg
Lundy PM, Tremblay KP (1979) Ganglion blocking properties of some bispyridinium soman antagonists. Eur J Pharmacol 60: 47–53
Lundy PM, Hansen AS, Hand BT, Boulet CA (1992) Comparison of several oximes against poisoning by soman, tabun and GF. Toxicology 72: 99–105
Lüttringhaus A, Hagedorn I (1964) Quartäre Hydroxyiminomethyl-pyridinium-salze. Das Dichlorid des Bis-[4-hydroxyiminomethyl-pyridinium-(1)-methylläthers] (“LüH 6”), ein neuer Reaktivator der durch organische Phosphorsäureester gehemmten Acetylcholinesterase. Arzneimittelforschung 14: 1–5
Marquardt DW (1963) An algorithm for least-square estimation on nonlinear parameters. J Soc Industr Appl Math 11: 431–441
Moncada S, Palmer MJ, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142
de la Motte S, Szinicz L (1991) Effects of pyridinium, 1-[[[4-(carbamoyl)-pyridinio]methoxy]methyl]-2-(hydroxyiminomethyl) dichloride monohydrate (HI 6) and atropine on the circulation and respiration of anaesthetized guinea-pigs. Arch Toxicol Suppl 14: 266–268
Nenner M (1970) Gleichzeitige Bestimmung der Aktivität von Acetylcholinesterase in Vollblut, Plasma und Erythrozyten mit dem automatischen Titrator. Z Klin Chem Klin Biochem 8: 537–540
Nenner M (1974) Phosphonylierte Aldoxime. Hemmwirkung auf Acetylcholinesterase und hydrolytischer Abbau. Biochem Pharmacol 23: 1255–1262
Oldiges H, Schoene K (1970) Pyridinium- und Imidazoliniumsalze als Antidote gegenüber Soman- und Paraoxonvergiftungen bei Mäusen. Arch Toxicol 26: 293–305
Queguiner G, Pastour P (1964) Preparation of pyridinedicarboxaldehydes. Compt Rend 258: 5903–5906
Queguiner G, Pastour P (1969) Synthesis in the pyridine series. V. Study of the reduction of ethyl pyridinedicarboxylates. Bull Soc Chim Fr 10: 3678–3683
Rand MJ, McCulloch MW, Story DT (1980) Catecholamine receptors on nerve terminals. In: Szekeris L (ed) Adrenergic activators and inhibitors. Part I. Handbook of experimental pharmacology, vol 54/I. Springer Berlin Heidelberg New York, pp 223–266
Reddy VK, Deshpande SS, Cintra WM, Scoble GT, Albuquerque EX (1991) Effectiveness of oximes 2-PAM and HI 6 in recovery of muscle function depressed by organophosphate agents in the rat hemidiaphragm: an in vitro study. Fundam Appl Toxicol 17: 746–760
Reithmann C, Arbogast H, Hallek M, Auburger G, Szinicz L (1988) Studies on the role of central catecholaminergic mechanisms in the antidotal effect of the oxime HI 6 in soman poisoned mice. Arch Toxicol 62: 41–44
Remien J, Mellinghoff A, Reithmeier I (1991) Muscarinic and nicotinic actions of oximes. Akademie Symposium: Role of oximes in the treatment of anticholinesterase agent poisoning, Munich, Oct 7
Rowland M, Tozer TN (1989) Clinical pharmacokinetics 2nd edn. Lea and Febiger, Philadelphia
Sachs L (1978) Angewandte Statistik 5. Aufl. Springer-Verlag Berlin Heidelberg New York
Schlagmann C, Ulbrich H, Remien J (1990) Bispyridinium (oxime) compounds antagonize the “ganglion blocking” effect of pyridostigmine in isolated superior cervical ganglia of the rat. Arch Toxicol 64: 482–489
Schoene K (1973) Phosphonyloxime aus Soman; Bildung und Reaktion mit Acetylcholinesterase in vitro. Biochem Pharmacol 22: 2997–3003
Schoene K, Oldiges H (1973) Die Wirkungen von Pyridiniumsalzen gegenüber Tabun- und Somanvergiftungen in vivo und in vitro. Arch Int Pharmacodyn 204: 110–123
Schoene K, Strake EM (1971) Reaktivierung von Diäthylphosphoryl-Acetylcholinesterase. Affinität und Reaktivität einiger Pyridiniumoxime. Biochem Pharmacol 20: 1041–1051
Sidell FR, Groff WA, Kaminskis A (1972) Toxogonin and pralidoxime: kinetic comparison after intravenous administration to man. J Pharm Sci 60: 860–863
Simons KJ, Briggs CJ (1983) The pharmacokinetics of HI 6 in beagle dogs. Biopharm Drug Dispos 4: 375–388
Stark I (1968) Versuche zur Darstellung eines LüH6 (Toxogonin) überlegenen Acetylcholinesterase-Reaktivators. Dipl. Arbeit, Univ. Freiburg
Stark I (1971) Reaktivierung phosphorylierter Acetylcholinesterase mit quaternierten Pyridinaldoximen. Ermittlung des Zusammenhangs zwischen Oximacidität und Reaktivierungsvermögen. Thesis, Univ. Freiburg
Steinberg GM, Solomon S (1966) Decomposition of a phosphonylated pyridinium aldoxime in aqueous solution. Biochemistry 5: 3142–3150
Weger N, Szinicz L (1981) Therapeutic effects of new oximes, benactyzine and atropin in soman poisoning: Part I. Effects of various oximes in soman, sarin, and Vx poisoning in dogs. Fundam AppI Toxicol 1: 161–163
Wolthuis OL, Cohen EM (1967) The effects of P2S, TMB-4, and LüH 6 on the rat phrenic nerve diaphragm preparation treated with soman or tabun. Biochem Pharmacol 16: 361–367
Wolthuis OL, Vanwersch RAP, van der Wiel HJ (1981) The efficacy of somebis-pyridinium oximes as antidotes to soman in isolated muscles of several species including man. Eur J Pharmacol 70: 355–369
Author information
Authors and Affiliations
Additional information
Part of thesis
Rights and permissions
About this article
Cite this article
Eyer, P., Hagedorn, I., Klimmek, R. et al. HLö 7 dimethanesulfonate, a potent bispyridinium-dioxime against anticholinesterases. Arch Toxicol 66, 603–621 (1992). https://doi.org/10.1007/BF01981499
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01981499