Summary
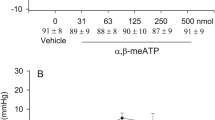
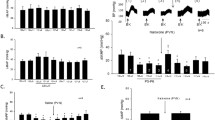
The effects of microinjecion of opioid receptor agonist and antagonist into mesencephalic nucleus dorsalis raphe, were studied on mean arterial pressure and heart rate to elucidate the nature and role of these opioid receptors in cardiovascular regulation. Microinjection of morphine (5 μg and 10 μg) into nucleus dorsalis raphe elicited both inhibitory and excitatory cardiovascular responses respectively, while microinjection of opioid receptor antagonist, naloxone (10 μg) failed to produce any significant cardiovascular responses. However, local pretreatment with naloxone blocked both inhibitory and excitatory responses of graded doses of morphine. These opioid receptors seem to be localised in the neurons of the nucleus since microinjection of morphine into neural structures adjoining nucleus dorsalis raphe failed to induce any cardiovascular responses. Furthermore, the dose or morphine (2 μg) which was ineffective when microinjected into nucleus dorsalis raphe, produced inhibitory cardiovascular responses after pretreatment with LM 5008, a 5-HT uptake blocker. Similarly, the excitatory cardiovascular responses of morphine microinjection were blocked by spinal cord transection (C1) and p-CPA, guanethidine and piperoxan pretreatments, while bilateral cervical vagotomy failed to do so. Thus, it is likely that the inhibitory cardiovascular responses of morphine are mediated directly through stimulation of opioid receptors present in the neurons of nucleus dorsalis raphe while the excitatory responses to higher dose of morphine, appear to be due to a release of noradrenaline which in turn modulates the activity of neurons by acting on a adrenoceptors.
Similar content being viewed by others
References
Aghajanian GK, Wang RY (1977) Habenular and other mid brain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res 122:229–242
Aghajanian GK, Wang RY, Baraban J (1978) Serotonergic and nonserotonergic neurons of the dorsal raphe: reciprocal changes in firing induced by peripheral nerve stimulation. Brain Res 153:169–175
Aiello-Malemberg P, Bartolini A, Bartolini R, Galli A (1979) Effects of morphine, physostigmine and raphe nuclei stimulation on 5-hydroxytryptamine release from the cerebral cortex of the cat. Br J Pharmacol 65:547–555
Anden NE, Dahlstrom A, Fuxe K, Larsson K, Olson L, Ungerstedt U (1966) Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol Scand 67:313–326
Bapeschi R, Theiss P, Herz A (1975) Effects of morphine on the turnover of catecholamines and serotonin in rats. Acute morphine administration. Eur J Pharmacol 34:253–261
Belin MF, Aguera M, Tappaz M, McRae-Degueurce A, Bobbilier P, Pujol JF (1979) GABA-accumulating neurons in the nucleus raphe dorsalis and periaqueductal gray in the rat: a biochemical and radioautographic study. Brain Res 170:279–297
Behbehani MM, Pomeroy SL (1978) Effect of morphine injected in periaqueductal gray on the activity of single units in nucleus raphe magnus of the rat. Brain Res 149:266–269
Cabot JB (1979) Raphe inhibition of sympathetic preganglionic neurons. Science 203:184–186
Dahlstrom A, Fuxe K (1964)Evidence for the existence of monoamine neurons in the central nervous system. 1. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand 62:(Suppl 232) 5–55
Dahlstrom A, Fuxe K (1965) Evidence for the existence of monoamine neurons in the central nervous system. 11. Experimentally induced changes in the intraneuronal amine levels of bulbospinal neurons systems. Acta Physiol Scand 64: (Suppl 247) 1–36
Fur GL, Uzan A (1977) Effects of 4-(3-indolyl-alkyl)piperidine derivatives on uptake and release of noradrenaline, dopamine, 5-hydroxytryptamine in rat brain synaptosomes, rat heart and human blood platelets. Biochem Pharmacol 26:497–503
Fuxe K, Jonsson G (1974) Further mapping of central 5-hydroxytryptamine neurons: Studies with the neurotoxic dihydroxytryptamine. In: Costa E, Gessa GL, Sandler M (eds) Serotonin, new vistas. Raven, New York, pp 1–12
Gent JP, Wolstencroft JH (1976) Actions of morphine, enkephalin and endorphin on single neurons in the brain stem, including the raphe and the periaqueductal gray of the cat. In: Opiates and endogenous opioid peptides. Elsevier/North-Holland Biomedical Press, Amsterdam, pp 217–224
Hokfelt T, Ljungdahl A, Terenius L, Elde R, Nilsson G (1977) Immunohistochemical analysis of peptide pathways possibly related to pain and analgesia: enkephalin and substance P. Proc Natl Acad Scie USA 74:3081–3085
Kee BK, Weissman A (1966) p-Chlorophenylalanine: A specific depletor of brain serotonin. J Pharmacol Exp Ther 154:499–516
Mohrland JS, Gebhart GF (1980) Effects of local electrical stimulation and morphine microinjection in the periaqueductal gray of the rat mesencephalon on neuronal activity in the medullary reticular formation. Brain Res 201:23–37
Neumayr RJ, Hare BD, Franz DN (1974) Evidence for bulbospinal control of sympathetic pregnaglionic neurons by monoaminergic pathways. Life Sci 14:793–806
Sakai K, Touret M, Salvert D, Leger L, Jouvet M (1977) Afferent projections to the cat locus coeruleus as visualized by the horseradish peroxidase technique. Brain Res 119:21–24
Saxena AK, Pant KK, Saksena AK, Tangri KK, Vrat S, Bhargava KP (1983) Cardiovascular responses elicited by microinjection of cholinergic agents into nucleus dorsalis raphe in cats. Clinical and Experimental Pharmacol Physiol 107:621–628
Saxena AK, Saksena AK, Agnihotri MS, Vrat S, Tangri KK, Bhargava KP (1985) Cardiovascular responses elicited by microinjection of monoamines into mesencephalic nucleus dorsalis raphe in cats. Naunyn-Schmiedeberg's Arch Pharmacol 329:141–145
Segal M (1979) Serotonergic innervation of the locus coeruleus from the dorsal raphe and its actions on responses to noxious stimuli. J Physiol 286:401–415
Smits JFM, Van Essen H, Struyker-Boudier HAJ (1978) Serotonin mediated cardiovascular responses to electrical stimulation of the raphe nuclei in the rat. Life Sci 23:173 -178
Snider RS, Niemer WT (1962) A stereotaxic atlas of the cat brain. The University of Chicago Press, Chicago
Way EL, Shen F (1971) Catecholamines and 5-hydroxytryptamine. In: Clout DH (ed) Narcotic drugs: Biochemical pharmacology. Plenum, New York, pp 229–253
Yaksh TL, Plant RL, Rudy TA (1977) Studies on the antagonism by raphe lesions of the antinociceptive action of systemic morphine. Eur J Pharmacol 41:399–408
Yarbrough GG, Buxbaum DM, Sanders-Bush E (1973) Biogenic amines and narcotic effects. II. Serotonin turnover in the rat after acute and chronic morphine administration. J Pharmacol Exp Ther 185:328–335
Author information
Authors and Affiliations
Additional information
Send offprint requests to K. K. Tangri at the above address
Rights and permissions
About this article
Cite this article
Saxena, A., Saksena, A., Vrat, S. et al. Presence of opioid receptors in mesencephalic nucleus dorsalis raphe concerned in cardiovascular regulation in cats. Naunyn-Schmiedeberg's Arch Pharmacol 336, 81–86 (1987). https://doi.org/10.1007/BF00177755
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00177755