Abstract
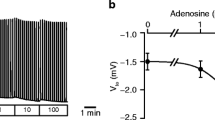
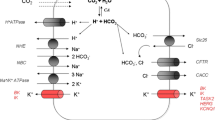
The aim of the present study was to investigate by what transport mechanism does HCO −3 cross the luminal membrane of pancreatic duct cells, and how do the cells respond to stimulation with dibytyryl cyclic AMP (db-cAMP). For this purpose a newly developed preparation of isolated and perfused intra-and interlobular ducts of rat pancreas was used. Responses of the epithelium to inhibitors and agonists were monitored by electrophysiological techniques. Addition of HCO −3 /CO2 to the bath side of nonstimulated ducts depolarized the PD across the basolateral membrane (PDbl) by about 9mV, as also observed in a previous study [21]. This HCO −3 effect was abolished by Cl− channel blockers or SITS infused into the lumen of the duct: i. e. 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB, 10−5 M) hyperpolarized PDbl by 8.2±1.6 mV (n=13); 3′,5-dichlorodiphenylamine-2-carboxylic acid (DCl-DPC, 10−5 M) hyperpolarized PDbl by 10.3±1.7 mV (n=10); and SITS hyperpolarized PDbl by 7.8±0.9 mV (n=4). Stimulation of the ducts with dbcAMP in the presence of bath HCO −3 /CO2 resulted in depolarization of PDbl, the ductal lumen became more negative and the fractional resistance of the luminal membrane decreased. Together with forskolin (10−6 M), db-cAMP (10−4 M) caused a fast depolarization of PDbl by 33.8±2.5 mV (n=6). When db-cAMP (5×10−4 M) was given alone in the presence of bath HCO −3 /CO2, PDbl depolarized by 25.3±4.2 mV (n=10). In the absence of exogenous HCO −3 , db-cAMP also depolarized PDbl by 24.7±3.0 mV (n=10). The present data suggest that in the luminal membrane of pancreatic duct cells there is a Cl− conductance in parallel with a Cl−/HCO −3 antiport. Dibutyryl cyclic AMP increases the Cl− conductance of the luminal membrane. Taking together our present results, and the recent data obtained for the basolateral membrane [21], a tentative model for pancreatic HCO −3 transport is proposed.
Similar content being viewed by others
References
Argent BE, Arkle S, Cullen MJ, Green R (1986) Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Q J Exp Physiol 71:633–648
Case RM, Argent BE (1986) Bicarbonate secretion by pancreatic duct cells: Mechanism and control. In: Go VLW, Gadner JD, Brooks FP, Lebethal E, Di Magno EP, Scheele GA (eds) The exocrine pancreas: Biology, pathology and diseases. Raven Press, New York, pp 213–243
Case RM, Scratcherd T (1972) The actions of dibutyryl cyclic adenosine 3′,5′-monophosphate and methyl xanthines on pancreatic exocrine secretion. J Physiol (Lond) 223:649–667
Case RM, Scratcherd T, Wynne RD'A (1970) The origin and secretion of pancreatic juice bicarbonate. J Physiol (Lond) 210:1–15
De Pont JJHHM, Luyben D, Bonting SL (1979) Rat pancreas adenylate cyclase. VI. Role of the enzyme in secretin stimulated enzyme secretion. Biochim Biophys Acta 584:33–42
De Pont JJHHM, Jansen JWCM, Kuijpers GAJ, Bonting SL (1982) A model for pancreatic fluid secretion. In: Case RM, Garner A, Turnberg LA, Young JA (eds) Electrolyte and water transport across gastrointestinal epithelia. Raven Press, New York, pp 11–20
Fölsch UR, Fischer H, Söling HD, Creutzfeldt W (1980) Effects of gastrointestinal hormones and carbamylcholine on cAMP accumulation in isolated pancreatic duct fragments from the rat. Digestion 20:277–292
Gögelein H, Greger R (1986) A voltage-dependent ionic channel in the basolateral membrane of late proximal tubules of the rabbit kidney. Pflügers Arch 407:S142-S148
Greenwell JR (1975) The effects of cholecystokininpancreozymin, acetylcholine and secretin on the membrane potentials of mouse pancreatic cells in vitro. Pflügers Arch 353:159–170
Greger R (1981) Cation selectivity of the isolated perfused cortical thick ascending limb of Henle's loop of rabbit kidney. Pflügers Arch 390:30–37
Greger R (1987) Role of K+ conductive pathways in the nephron. Kidney Int 31:1055–1064
Greger R (1988) Chloride channel blockers. In: Fleischer B, Fleischer S (eds) Methods in enzymology, vol. 5. Academic Press, New York London (in press)
Greger R, Schlatter E (1983) Properties of the lumen membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflügers Arch 396:315–324
Greger R, Schlatter E, Wang F, Forrest JN Jr (1984) Mechanism of NaCl secretion in rectal gland tubules of spiny dogfish (Squalus acanthias). III. Effects of stimulation of secretion by cyclic AMP. Pflügers Arch 402:376–384
Kanno T, Yamamoto M (1977) Differentiation between the calcium-dependent effects of cholecystokinin-pancreozymin and the bicarbonate-dependent effects of secretin in exocrine secretion of the rat pancreas. J Physiol (Lond) 264:787–790
Kimura T, Imamura K, Eckhardt L, Schulz I (1987) Ca2+-, phorbol ester-, and cAMP-stimulated enzyme secretion from permeabilized rat pancreatic acini. Am J Physiol 250:G698-G708
Kuijpers GAJ, Van Nooy IGP De Pont JJHHM, Bonting SL (1984) The mechanism of fluid secretion in the rabbit pancreas studied by means of various inhibitors. Biochim Biophys Acta 778:324–331
Lingard JM, Young JA (1983) β-Adrenergic control of exocrine secretion by perfused rat pancreas in vitro. Am J Physiol 245:G690-G696
Novak I, Greger R (1987) Effect of inhibitors and ion substitutions on PDbl of pancreatic duct cells. Pflügers Arch 408:R48
Novak I, Greger R (1987) Cellular mechanism of bicarbonate transport in isolated perfused pancreatic ducts. Acta Physiol Scand 129:13A
Novak I, Greger R (1988) Electrophysiological study of transport systems in isolated perfused pancreatic ducts. Properties of the basolateral membrane. Pflügers Arch 411:58–68
Petersen OH (1986) Calcium-activated potassium channels and fluid secretion by exocrine glands. Am J Physiol 251:G1-G13
Petersen OH, Findlay I (1987) Electrophysiology of the pancreas. Physiol Rev 67:1054–1116
Petersen OH, Ueda N (1977) Secretion of fluid and amylase in the perfused rat pancreas. J Physiol (Lond) 264:819–835
Reuss L, Constantin JL, Bazile JE (1987) Diphenylamine-2-carboxylate blocks Cl−/HCO −3 exchange inNecturus gallbladder epithelium. Am J Physiol 253:C79-C89
Schulz I (1971) Influence of bicarbonate-CO2-and glycodiazine buffer on the secretion of the isolated cat's pancreas. Pflügers Arch 329:283–306
Schulz I (1980) Bicarbonate transport in the exocrine pancreas. Ann NY Acad Sci 341:191–209
Schulz I (1987) Electrolyte and fluid secretion in the exocrine pancreas. In: Johnson LR (ed) Physiology of gastrointestinal tract, vol. 1. Raven Press, New York, pp 1147–1171
Schulz I, Ströver, F, Ullrich KJ (1971) Lipid soluble weak organic acid buffers as “substrate” for pancreatic secretion. Pflügers Arch 323:121–140
Scratcherd T, Hutson D (1982) The role of chloride in pancreatic secretion. In: Case RM, Garner A, Turnberg LA, Young JA (eds) Electrolyte and water transport across gastrointestinal epithelia. Raven Press, New York, pp 61–69
Scratcherd T, Hutson D, Case RM (1981) Ionic transport mechanisms underlying fluid secretion by the pancreas. Phil Trans R Soc Lond B 296:167–178
Seow F, Young JA (1984) Anionic dependency of secretinstimulated secretion by the isolated perfused rat pancreas. In: Case RM, Lingard JM, Young JA (eds) Secretion: mechanism and control. Manchester University Press, Manchester, pp 97–99
Seow KTF, Lingard JM, Young JA (1986) Anionic basis of fluid secretion by rat pancreatic acini in vitro. Am J Physiol 250:G140-G148
Sewell WA, Young JA (1975) Secretion of electrolytes by the pancreas of the anaesthetized rat. J Physiol (Lond) 252:379–396
Swanson CH, Solomon AK (1972) Evidence for Na−H exchange in the rabbit pancreas. Nature 236:183–184
Swanson CH, Solomon AK (1975) Micropuncture analysis of the cellular mechanism of electrolyte secretion by the in vitro rabbit pancreas. J Gen Physiol 65:22–45
Trimble ER, Bruzzone R, Biden TJ, Farese RV (1986) Secretin induces rapid increases in inositol triphosphate, cytosolic Ca2+ and diacylglycerol as well as cyclic AMP in rat pancreatic acini. Biochem J 239:257–261
Wangemann P, Wittner M, Di Stefano A, Englert HC, Lang HJ, Schlatter E, Greger R (1986) Cl−-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflügers Arch 407:S128-S141
Welsh MJ, Smith PL, Frizzell RA (1983) Chloride secretion by canine tracheal epithelium. III. Membrane resistances and electromotive forces. J Membr Biol 71:209–218
Yoshitomi K, Burckhardt B-Ch, Frömter E (1985) Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflügers Arch 405:360–366
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Novak, I., Greger, R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts. Pflugers Arch. 411, 546–553 (1988). https://doi.org/10.1007/BF00582376
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00582376