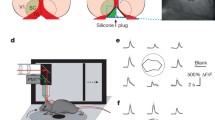
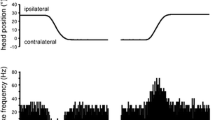
Summary
Cells with uniform receptive fields were selected for extra cellular recording in the striate cortex of anaesthetised cats. From their responses to electrical stimulation at three sites in the primary visual pathway the cells were grouped according to their ordinal position and whether their afferent drive came from the brisk sustained or brisk transient type of LGN neuron. From differences in laminar distribution and afferent stream the population was divided into 4 subgroups. Within these 4 subgroups there were two basic visual response patterns, which had been identified previously, and attributed to B and C cells. The B cells, which have a smaller receptive field, a lower spontaneous activity and cut-off velocity than C cells, were found to receive their input from slowly conducting afferents while the afferents to C cells arose from the fast stream. A high proportion of both B and C cells received a monosynaptic or direct drive from the optic radiations and responded with multiple spiking to a single electrical shock. Multiple spiking was viewed as evidence of secondary pathways travelling via intermediate cortical neurons to contribute to the cell's input. An examination of the visual properties of all subclasses showed that the more obvious differences in receptive field properties were associated with the type of afferent coming from the LGN rather than with the ordinal or the laminar position of the cell. In this respect the cells in the C/B family resemble S cells, whose receptive field properties also show a dependence on the type of LGN input.
Similar content being viewed by others
References
Albus K, Donate-Oliver F (1977) Cells of origin of the occipitopontine projection in the cat: Functional properties and intracortical location. Exp Brain Res 28: 167–174
Barlow HB, Blakemore C, Pettigrew JD (1967) The neural mechanisms of binocular depth discrimination. J Physiol (Lond) 193: 327–342
Bishop PO, Henry GH, Smith CJ (1971) Binocular interaction fields of single units in the cat striate cortex. J Physiol (Lond) 216: 39–68
Bishop PO, Kato H, Orban GA (1980) Direction-selective cells in complex family in cat striate cortex. J Neurophysiol 43: 1266–1283
Bullier J, Henry GH (1979a) Ordinal position of neurons in cat striate cortex. J Neurophysiol 42: 1251–1263
Bullier J, Henry GH (1979b) Neural path taken by afferent streams in striate cortex of the cat. J Neurophysiol 42: 1264–1270
Bullier J, Henry GH (1979c) Laminar distribution of first-order neurons and afferent terminals in cat striate cortex. J Neurophysiol 42: 1271–1281
Bullier J, Mustari MJ, Henry GH (1982) Receptive field transformations between LGN neurons and S cells of cat striate cortex. J Neurophysiol 47: 417–438
Cleland BG, Dubin MW, Levick WR (1971) Sustained and transient cells in the cat's retina and lateral geniculate nucleus. J Physiol (Lond) 217: 473–496
Cleland BG, Levick WR, Morstyn R, Wagner HG (1976) Lateral geniculate relay of slowly conducting retinal afferents to the cat visual cortex. J Physiol (Lond) 255: 299–320
Dreher B, Leventhal AG, Hale PT (1980) Geniculate input to cat visual cortex: A comparison of area 19 with area 17 and 18. J Neurophysiol 44: 804–826
Ferster D, LeVay S (1978) The axonal arborizations of lateral geniculate neurons in the striate cortex of the cat. J Comp Neurol 182: 923–944
Fukuda Y, Stone J (1974) Retinal distribution and central projections of Y-, X-, and W-cells of the cats retina. J Neurophysiol 37: 749–772
Gilbert CD (1977) Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol (Lond) 268: 391–422
Gilbert CD, Kelly JP (1975) The projections of cells in different layers of the cat's visual cortex. J Comp Neurol 163: 81–106
Goodwin AW, Henry GH (1975) Direction selectivity of complex cells in a comparison with simple cells. J Neurophysiol 38: 1524–1540
Harvey AR (1980) A physiological analysis of subcortical and commissural projections of areas 17 and 18 of the cat. J Physiol (Lond) 302: 507–534
Henry GH, Bishop PO, Tupper RM, Dreher R (1973) Orientation specificity and response variability of cells in the striate cortex. Vision Res 13: 1771–1779
Henry GH, Goodwin AW, Bishop PO (1978) Spatial summation of responses in receptive fields of single cells in cat striate cortex. Exp Brain Res 32: 245–266
Henry GH, Lund JS, Harvey AR (1978) Cells of the striate cortex projecting to the Clare-Bishop area of the cat. Brain Res 151: 154–158
Hoffmann KP, Stone J, Sherman SM (1972) Relay of receptive field properties in dorsal lateral geniculate nucleus of the cat. J Neurophysiol 35: 518–531
Hubel DH, Wiesel TN (1962) Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol (Lond) 160: 106–154
Kulikowski JJ, Bishop PO (1982) Silent periodic cells in the cat striate cortex. Vis Res 22: 191–200
LeVay S, Gilbert CD (1976) Laminar patterns of geniculocortical projection in the cat. Brain Res 113: 1–19
Levick WR (1972) Another tungsten microelectrode. Med Biol Eng 10: 510–515
Mustari MJ, Bullier J, Henry GH (1982) Comparison of the response properties of three types of monosynaptic S cell in cat striate cortex. J Neurophysiol 47: 439–454
Palmer LA, Rosenquist AC (1974) Visual receptive fields of single striate cortical units projecting to the superior colliculus in the cat. Brain Res 67: 27–42
Singer W, Tretter F, Cynader M (1975) Organization of cat striate cortex: A correlation of receptive-field properties with afferent and efferent connections. J Neurophysiol 38: 1080–1098
Sillito AM (1977) Inhibitory processes underlying the directional specificity of simple, complex an hypercomplex cells in the cat's visual cortex. J Physiol (Lond) 271: 699–720
Wolbarsht ML, MacNichol EF, Jr, Wagner HG (1960) Glass insulated platinum electrodes. Science 132: 1309–1310
Wassle H, Boycott BB, Illing R-B (1981) Morphology and mosaic of on- and off-beta cells in the cat retina and some functional considerations. Proc R Soc Lond B 212: 177–195
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Henry, G.H., Mustari, M.J. & Bullier, J. Different geniculate inputs to B and C cells of cat striate cortex. Exp Brain Res 52, 179–189 (1983). https://doi.org/10.1007/BF00236626
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00236626