Abstract
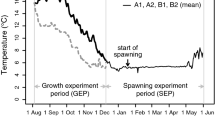
Samples of juveniles and adults of the goby Pseudogobius olorum were collected from seven sites in the shallows of the upper Swan Estuary, Western Australia, using a 3 mm-mesh seine net on one or two occasions in each month between September 1983 and April 1985. The mean gonadosomatic index of female fish rose from very low values in winter (June–August) to a sharp peak in mid-spring (October), reflecting the rapid maturation of ovaries over, this period. Ovaries with post-ovulatory follicles and ovaries that were undergoing degeneration were present, in November and December, but were then either rare or absent in those members of the corresponding cohort which survived into January and February. Female fish with advanced oocytes and mature ovaries were not found in December and January, but were present in February to April. The above trends exhibited by ovarian maturity indices, together with the appearance of larvae and small fish in both spring and autumn, demonstrate that P. olorum spawns in both spring and autumn and at best to only a limited extent in summer. Length-frequency and gonadal data show that the progeny of the spring-spawning group frequently spawn in the following autumn, when they are ∼ 5 mo old, and that those of the autumn-spawning group frequently spawn in the following spring, when they are ∼ 7 mo old. Some representatives of these two spawning groups survive through the winter and summer, respectively, to breed in a second season. Growth of the progeny of the spring-spawning group was relatively rapid between late spring and mid-autumn, whereas that of the autumn-spawning group was negligible during winter, but then inceased markedly in spring. It is proposed that the biannual spawning periods in each year by P. olorum in the Swan Estuary developed as a result of a rise in water temperature over the last few thousand years. Such a rise would have brought forward further into spring and extended later into autumn the periods when the water temperatures lie within the range (20 to 25°C) at which P. olorum typically spawns. However, mid-summer is now characterised by water temperatures >25°C, which are considered less conducive to reproductive success.
Similar content being viewed by others
References
Allen GR (1989) Freshwater fishes of Australia. TFH, Neptune City
Barlow GW, De Vlamingh VL (1972) Ovarian cycling in longjaw gobies, Gillichthys mirabilis, from the Salton Sea. Calif Fish Game 58: 50–57
Birdsong RS, Murdy EO, Pezold FL (1988) A study of the vertebral column and median fin osteology in gobioid fishes with comments on gobioid relationships. Bull mar Sci 42: 174–214
Chalmer PN, Hodgkin EP, Kendrick GW (1976) Benthic faunal changes in a seasonal estuary of south-western Australia. Rec West Aust Mus 4: 383–410
Chrystal PJ, Potter IC, Loneragan NR, Holt CP (1985) Age structure, growth rates, movement patterns and feeding in an estuarine population of the cardinalfish Apogon rueppellii. Mar Biol 85: 185–197
Claridge PN, Hardisty MW, Potter IC, Williams CV (1985) Abundance, life history and ligulosis, in the gobies (Teleostei) of the inner Severn Estuary. J mar biol Ass UK 65: 951–968
Cole KS, Robertson DR, Cedeno AA (1994) Does gonad structure reflect sexual pattern in all gobiid fishes? Envir Biol Fish 41: 301–309
Darcy GH (1980) Comparison of ecological and life history information on gobiid fishes, with emphasis on the southeastern United States. NOAA natn mar Fish Serv tech Memo US Dep Commerce SEFC 15: 1–55
Day JH (1981) The estuarine fauna. In: Whitfield AK (ed) Estuarine ecology with particular reference to Southern Africa. A.A. Balkema, Rotterdam, pp 197–221
De Vlamingh VL (1972a) Reproductive cycling in the estuarine gobiid fish, Gillichthys mirabilis. Copeia 1972: 278–291
De Vlamingh VL (1972b) The role of the endocrine system in temperature-controlled reproductive cycling in the estuarine gobiid fish, Gillichthys mirabilis. Comp Biochem Physiol 41A: 697–713
Dotu Y (1958) The bionomics and life history of two gobioid fishes, Tridentiger undicervicus Tomiyama and Tridentiger trigonocephalus (Gill) in the innermost part of Ariake Sound. Sci Bull Fac Agric Kyushu Univ 16: 343–358
Founds M (1973) Sand gobies in the Dutch Wadden Sea (Pomatoschistus, Gobiidae, Pisces). Neth J Sea Res 6: 417–478
Fouda MM, Hanna MY, Fouda FM (1993) Reproductive biology of a Red Sea goby, Silhouettea aegyptia, and a Mediterranean goby, Pomatoschistus marmoratus, in Lake Timash, Suez Canal. J Fish Biol 43: 139–151
Fouda MM, Miller PJ (1981) Age and growth of the common goby, Pomatoschistus microps, on the south coast of England. Estuar, cstl Shelf Sci 12: 121–129
Geevarghese C, John PA (1983) Maturation and spawning of a gobiid fish, Oligolepis acutipennis (Cuv. and Val.), from the south-west coast of India. J Fish Biol 23: 611–624
Gibson RN (1970) Observations on the biology of the giant goby Gobius cobitis Pallas. J Fish Biol 2: 281–288
Gibson RN, Ezzi IA (1978) The biology of a Scottish population of Fries' goby, Lesueurigobius friesii. J Fish Biol 12: 371–389
Gibson RN, Ezzi IA (1981) The biology of the Norway goby, Pomatoschistus norvegicus (Collett), on the west coast of Scotland. J Fish Biol 19: 697–714
Gill HS, Potter IC (1993) Spatial segregation amongst goby species within an Australian estuary, with a comparison of the diets and salinity tolerance of the two most abundant species. Mar Biol 117: 515–526
Giulianini PG, Ferrero EA, Patzner R, Di Marcotullio A (1992) Ovarian cycle in Zosterisessor ophiocephalus (Osteichthyes, Gobiidae). Oebalia 1992 (Suppl XVII): 141–142
Giulianini PG, Di Marcotullio A, Ferrero EA, Patzner R (1994) Light microscopical and ultrastructural cytology of the ovaries in the sea-grass goby, Zosterisessor ophiocephalus (Osteichthyes, Gobiidae). Boll Zool 61: 135–144
Grossman GD, Coffin R, Moyle PB (1980) Feeding ecology of the bay goby (Pisces: Gobiidae),. Effects of behavioural, ontogenetic, and temporal variation on diet. J exp mar Biol Ecol 44: 47–59
Halse SA (1981) Faunal assemblages of some saline lakes near Marchagee, Western Australia. Aust J mar Freshwat Res 32: 133–142
Healey MC (1971a) The distribution and abundance of sand gobies, Gobius minutus, in the Ythan estuary. J Zool, Lond 163: 177–229
Healey MC (1971b) Gonad development and fecundity of the sand goby, Gobius minutus Pallas. Trans Am Fish Soc 100: 520–526
Hesthagen IH (1975) Seasonal occurrence and length variation in the sand goby, Pomatoschistus minutus (Pallas), in the shore zone of the inner Oslofjord. Norw J Zool 23: 235–242
Hesthagen IH (1977) Migrations, breeding, and growth in Pomatoschistus minutus (Pallas) (Pisces: Gobiidae) in Oslofjorden, Norway, Sarsia 63: 17–26
Hoese DF (1994) Gobies. In: Paxton JR, Eschmeyer WN (eds) Encyclopedia of fishes. University of New South Wales Press, Sydney, pp 220–224
Humphries P, Hyndes GA, Potter IC (1992) Comparisons between the diets of distant taxa (teleost and cormorant) in an Australian estuary. Estuaries 15: 327–334
Kimura DK (1980) Likelihood methods for the von Bertalanffy growth curve. Fish Bull US 77: 765–776
Khoo KH (1979) The histochemistry and endocrine control of vitellogenesis in goldfish ovaries. Can J Zool 57: 617–626
Laevastu T (1965) Manual of methods in fishery biology. Fascicule 9. Section 4. Research on fish stocks. 1. FAO Rome (FAO Man Fish Sci)
Last PR, Scott EOG, Talbot FH (1983) Fishes of Tasmania.. Tasmanian Fisheries Development Authority, Hobart., Tasmania
Lindström K, Ranta E (1992) Predation by birds affects population structure in breeding sand goby, Pomatoschistus, males. Oikos 64: 527–532
Loneragan NR, Potter IC, Lenanton RCJ (1989) Influence, of site, season and year on contributions made by marine, estuarine, diadromous and freshwater species to the fish fauna of a temperate Australian estuary. Mar Biol 103: 461–479
Macdonald PDM, Green PEJ (1990) User's guide to a program MIX (Release 3): an interactive program for fitting mixtures of distributions. Ichthus Data Systems. Ontario, Canada
Macdonald PDM, Pitcher TJ (1979) Age-groups from size-frequency data: a versatile and efficient method of analyzing distribution mixtures. J Fish Res Bd Can 36: 987–1001
Miller PJ (1975) Age-structure and life-span in the common goby Pomatoschistus microps. J Zool, Lond 177: 425–448
Miller PJ (1984) The tokology of gobioid fishes. In: Potts GW, Wootton RJ (eds) Fish reproduction: strategies and tactics. Academic Press, London, pp119–153
Miller PJ, Fouda MM (1986) Notes on the biology of a Red Sea goby, Silhouettea aegyptia (Chabanaud, 1933) (Teleostei: Gobiidae). Cybium 10: 395–409
Morcira F, Costa JL, Almeida PR, Assic C, Costa MJ (1991) Age determination in Pomatoschistus minutus (Pallas) and Pomatoschistus microps (Krøyer) (Pisces: Gobiidae) from the upper Tagus estuary, Portugal. J Fish Biol 39: 433–440
Neira FJ, Potter IC, Bradley JS (1992) Seasonal and spatial changes in the larval fish fauna within a large temperate Australian estuary. Mar Biol 112: 1–16
Nel SA, Potter IC, Loneragan NR (1985) The biology of the catfish Cnidoglanis macrocephalus (Plotosidae) in an Australian estuary. Estuar, cstl Shelf Sci 21: 895–909
Nielsen LA, Johnson DL (1983) Fisheries techniques. American Fisheries Society, Bethesda
Pauly D, Gaschutz G (1979) A simple method for fitting oscillating length growth data with a program for pocket calculators. Int Counc Explor Sea Comm Meet (Demersal Fish Comm) G 24: 1–26
Potter IC, Cheal AJ, Loneragan NR (1988) Protracted estuarine phase in the life cycle of the marine pufferfish Torquigener pleurogramma. Mar Biol 98: 317–329
Potter IC, Ivantsoff W, Cameron R, Minnard J (1986) Life cycles and distribution of atherinids in the marine and estuarine waters of southern Australia. Hydrobiologia 139: 23–40
Potter IC, Loneragan NR, Lenanton RCJ, Chrystal PJ, Grant CJ (1983) Abundance, distribution and age structure of fish populations in a Western Australian estuary. J Zool, Lond 200: 21–50
Potter IC, Neira FJ, Wise BS, Wallace JH (1994) Reproductive biology and larval development of the terapontid Amniataba caudavittata, including comparisons with the reproductive strategies of other estuarine teleosts in temperate, Western Australia. J Fish Biol 45: 57–74
Prince JD, Potter JC (1983) Life-cycle duration, growth and spawning times of five species of Atherinidae (Teleostei) found in a Western Australian estuary. Aust J mar Freshwat Res 34: 287–301
SPSS Inc. (1986) SPSSX user's guide. 2nd edn. McGraw-Hill, New York
Trayler KM, Brothers DJ, Wooller RD, Potter IC (1989) Opportunistic foraging by three, species of cormorants in an Australian estuary. J Zool, Lond 218: 87–98
Walker D (1978) Quaternary climates of the Australian region. In: Pittock AB, Frakes LA, Jensen D, Peterson JA, Zillman JW (eds) Climatic change and variability; a southern perspective. Cambridge University Press, Cambridge, pp 82–97
Wise BS, Potter IC, Wallace JH (1994) Growth, movements and diet of the terapontid Amniataba caudavittata in an Australian estuary. J Fish Biol 45: 917–931
Author information
Authors and Affiliations
Additional information
Communicated by G. F. Humphrey, Sydney
Rights and permissions
About this article
Cite this article
Gill, H.S., Wise, B.S., Potter, I.C. et al. Biannual spawning periods and resultant divergent patterns of growth in the estuarine goby Pseudogobius olorum: temperature-induced?. Marine Biology 125, 453–466 (1996). https://doi.org/10.1007/BF00353258
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00353258