Abstract
Objective: This study was undertaken in order to evaluate the impact of pharmacokinetics on the toxicity of oral etoposide administered daily for 21 days. Methods: The daily dose was 50 mg/m2. Thirty-two patients 24 males and eight females, 36–76 years old, treated for various tumour types), were evaluated. Blood samples were obtained on day 1 for all patients, and on day 21 for 16 patients. Plasma etoposide concentrations were determined by high-performance liquid chromatography, and etoposide plasma protein binding by equilibrium dialysis.
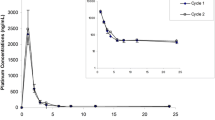
Results: On day 1, the mean value (with coefficient of variation for interindividual variability) for the unbound fraction (fu), area under the concentration versus time curve (AUC), and unbound AUC was 9.8% (59%), 34 mg · h/l (39%), and 3.5 mg · h/l (92%), respectively. The ratio between AUC on day 1 and day 21 ranged between 0.5 and 1.8 (mean 0.9, with CV 33%). The plasma trough unbound concentrations and the unbound AUCs both corresponding to the first administration were significantly higher in the 11 patients who had a severe neutropenia than in the 21 patients who had no or moderate toxicity. However, total etoposide concentrations did not differ between these two groups. A limited sampling strategy using the NONMEM program and a database of 89 patients previously studied was performed. The optimal sampling schedule (i.e. 1, 4, and 24 h after oral etoposide administration) allowed to obtain the AUC accurately on day 1.
Conclusion: Individual adjustment of oral etoposide based on unbound pharmacokinetics after the first administration appears relevant and feasible.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 29 June 1998 / Accepted in revised form: 8 September 1998
Rights and permissions
About this article
Cite this article
Perdaems, N., Bachaud, J., Rouzaud, P. et al. Relation between unbound plasma concentrations and toxicity in a prolonged oral etoposide schedule. E J Clin Pharmacol 54, 677–683 (1998). https://doi.org/10.1007/s002280050534
Issue Date:
DOI: https://doi.org/10.1007/s002280050534