Summary
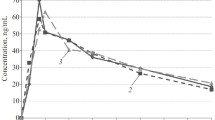
A method has been developed for the quantitative determination of heptabarbital [5-(1-cyclohepten-1-yl)-5-ethylbarbituric acid] in human plasma after administration of single therapeutic doses of the drug. It involves a single extraction step followed by gas chromatography with alkali flame ionization detection, and the results were linear in the concentration range 0.125 – 5.0 µg/ml plasma. The pharmacokinetics and relative bioavailability of heptabarbital and heptabarbital sodium were studied in a crossover design in 7 healthy volunteers after oral administration of 20 tablets containing 200 mg heptabarbital and hard gelatine capsules containing an equivalent amount of its sodium salt. Heptabarbital concentrations in plasma were determined at regular intervals. The absorption of heptabarbital from the tablets was quite slow and peak level times varied from 1.5 to 4 h. The sodium salt was absorbed more rapidly and peak concentrations occurred between 1/3 and 2 h. In all cases the elimination of heptabarbital could be described by a single first-order process with an average half-life of 7.6 h (range 6.1 – 11.2 h). The half-life of the drug in each individual was about the same in the two trials. The relative bioavailability in each volunteer was estimated by comparing the areas under the plasma concentration curves. The sodium salt had an average bioavailability of 83% relative to the free acid. In some volunteers urinary excretion of unchanged heptabarbital was measured; cumulative excretion amounted to 0.16 – 0.30% of the administered dose. Four volunteers received one tablet each night for eight or ten days, but no accumulation was found. In three volunteers the half-life of the drug prior to and after these experiments did not change, whereas in the other volunteer the half-life decreased from 7.1 to 4.6 h. The possibility of enzyme induction should be considered when heptabarbital is taken regularly. It was concluded that heptabarbital was a suitable drug for the treatment of insomnia, since its half-life was rather short. Heptabarbital sodium may be used for induction of sleep, whereas Medomin® tablets, i.e. heptabarbital free acid, may be prescribed when the maintenance of sleep is the primary reason for treatment with a hypnotic drug.
Similar content being viewed by others
References
Aggeler, P.M., O'Reilly, R.: Effect of heptabarbital on the response to bishydroxycoumarin in man. J. Lab. clin. Med.74, 229–238 (1969)
Atkinson, R.M., Bedford, C.B., Child, K.J., Tomich, E.G.: Effect of particle size on blood griseofulvin levels in man. Nature (Lond.)193, 588–590 (1962)
Balasubramaniam, K., Lucas, S.B., Mawer, G.E., Simons, P.J.: The kinetics of amylobarbitone metabolism in healthy men and women. Brit. J. Pharmacol.39, 564–572 (1970)
Barr, W.H.: Bioavailability of oral solid dosage forms and clinical response to drug therapy. In: Current concepts in the pharmaceutical sciences: dosage form design and bioavailability. Philadelphia: Lea & Febiger 1973
Benet, L.Z.: Biopharmaceutics as a basis for the design of drug products. In: Drug design, Vol. IV. New York: Academic Press 1973
Blake, M.I.: Role of compendia in controlling factors affecting bioavailability of drug products. J. Amer. pharm. Ass. NS11, 603–611 (1971)
Borgland, R.G., Nicholson, A.N.: Human performance after a barbiturate (heptabarbitone). Brit. J. clin. Pharmacol.1, 209–215 (1974)
Breimer, D.D., van Rossum, J.M.: Rapid and sensitive gas chromatographic determination of hexobarbital in plasma of man using a nitrogen detector. J. Chromatogr.88, 235–243 (1974)
Breimer, D.D.: Pharmacokinetics of hypnotic drugs. Studies on the pharmacokinetics and biopharmaceutics of barbiturates and chloral hydrate in man. Ph.D. thesis, University of Nijmegen, The Netherlands 1974
Breimer, D.D., Honhoff, C., Zilly, W., Richter, E., van Rossum, J.M.: Pharmacokinetics of hexobarbital in man after intravenous infusion. J. Pharmacokin. Biopharm.3, 1–11 (1975)
Butler, T.C.: Rate of penetration of barbituric acid derivatives into brain. J. Pharmacol. exp. Ther.100, 219–225 (1950)
Campagna, F.A., Cureton, G., Merigian, R.A., Nelson, E.: Inactive prednisone tablets U.S.P. XVI. J. pharm. Sci.52, 605–606 (1963)
Clifford, J.M., Cookson, J.H., Wickham, P.E.: Absorption and clearance of secobarbital, heptabarbital, methaqualone and ethinamate. Clin. Pharmacol. Ther.16, 376–389 (1974)
Dayton, P.G., Tarcan, Y., Chenkin, T., Weiner, M.: The influence of barbiturates on coumarin plasma levels and prothrombin response. J. clin. Invest.40, 1797–1802 (1961)
Doornbos, D.A., de Zeeuw, R.A.: The determination of the acid dissociation constants of barbiturates by an accurate method of pH measurement. Pharm. Weekblad104, 233–251 (1969)
Dost, F.H.: Grundlagen der Pharmakokinetik. Stuttgart: Georg Thieme Verlag 1968
Ehrnebo, M.: Pharmacokinetics and distribution properties of pentobarbital in humans following oral and intravenous administration. J. pharm. Sci.63, 1114–1118 (1974)
Fernandez-Guardiola, A., Lerdo de Tejada, A., Contreras, C., Salgado, A., Ayala, F.: Polygraphic study in man to differentiate sleep-inducing action of hypnotics. Psychopharmacologia (Berl.)26, 285–295 (1972)
Garrett, E.R.: The physicochemical and pharmacokinetic bases for the biopharmaceutical evaluation of drug biological availability in pharmaceutical formulations. Acta Pharmacol. (Kbh.)29, (Suppl. 3) 1–29 (1971)
Gilbert, J.N.T., Millard, B.J., Powell, J.W.: Gas chromatography-mass spectrometry for the identification of barbiturate metabolites. Brit. J. Pharmacol.47, 665 P (1973)
Goldstein, A., Aronow, L., Kalman, S.M.: Principles of drug action. New York: Harper & Row 1969
Higuchi, W.I., Parrott, E.L., Wurster, D.E., Higuchi, T.: Investigation of drug release from solids. II. Theoretical and experimental study of influences of bases and buffers on rates of dissolution of acidic solids. J. Amer. pharm. Ass. sci. Ed.47, 376–383 (1958)
Lienert, G.A.: Zum Thema: Beeinträchtigen Schlafmittel die psychische Leistung? Dtsch. Med. Wschr.79, 1180–1182 (1954)
Lienert, G.A.: Vergleichende pharmakophysiologische Untersuchungen über Schlafmittelwirkungen und -nachwirkungen. Medizinische2, 1608–1614 (1956)
Martindale: The Extra Pharmacopoeia. London: The Pharmaceutical Press 1972
Remmer, H.: Induction of drug metabolizing enzyme system in the liver. Europ. J. clin. Pharmacol.5, 116–136 (1972)
Sjögren, J.: Importance of pharmaceutical formulation for drug absorption. Acta pharmacol. (Kbh.)29, (Suppl. 3) 68–80 (1971)
Sjögren, J., Sölvell, L., Karlsson, I.: Studies on the absorption rate of barbiturate in man. Acta med. scand.178, 553–559 (1965)
Vesell, E.S.: Introduction: genetic and environmental factors affecting drug response in man. Fed. Proc.31, 1253–1269 (1972)
Wagner, J.G.: Biopharmaceutics and relevant pharmacokinetics. Hamilton: Drug Intelligence Publications 1971
Weithaler, K., Biedermann, G.: Klinische und experimentelle Untersuchungen über ein Schlafmittel. Med. Klinik50, 2155–2158 (1955)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Breimer, D.D., de Boer, A.G. Pharmacokinetics and relative bioavailability of heptabarbital and heptabarbital sodium after oral administration to man. Eur J Clin Pharmacol 9, 169–178 (1975). https://doi.org/10.1007/BF00614014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00614014