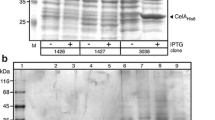
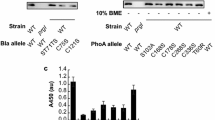
Abstract
In our studies of structure-function correlation of polypeptides we used Tendamistat (TM), an α-amylase-inhibitor of Streptomyces tendae, as a model to investigate the influence of different mutants on the expression and secretion of the protein. In addition, we examined the influence of replacing the two disulphide-bridges that stabilize the two-loop structure of the whole protein. The single mutants C27S, C27T, C45A, the double mutants C11A/C27A, C11A/C27S, C11A/C27T, C11A/C27L, C45/C73A and the fourfold mutant C11A/C27A/C45A/C73A were prepared. The mutated TM gene was expressed in S. lividans TK 24, which secretes the active form of the inhibitor into the culture medium. Compared with the wild-type, the double-mutated TM derivatives show an increase in secreted protein by a factor of two to ten. In contrast, the single-mutated inhibitor analogues show the reverse effect. In order to examine the influence of temperature and culture media on the production of protein derivative we used the most unstable C11A analogue. Our expression studies at 10, 19, 28 and 37° C established 19° C as the optimal temperature for production of the protein derivatives. The correlation between the stability and secretion of TM is discussed in the context of our knowledge of protein translocation in bacteria. Based on these experiments we optimized the fermentation parameters, isolated TM analogous on a large scale, and verified them.
Similar content being viewed by others
References
Aretz W, Koller KP, Riess G (1989) Proteolytic enzymes from recombinant Streptomyces lividans TK 24. FEMS Lett 65:31–36
Aschauer H, Vértesy L, Braunitzer G (1981) Die Sequenz des α-Amylaseinhibitors Hoe-467 A aus Streptomyces tendae. Hoppe-Seyler's Z Physiol Chem 362:465–467
Bender E, Koller K-P, Engels J (1990) Secretory expression of interleukin-2 by Streptomyces lividans. Gene 86:227–232
Bergmeyer HU (1970) Methoden der enzymatischen Analyse, vol 1, 2nd edn. Verlag Chemie, Weinheim, pp 394–395
Bieker KL, Silhavy TJ (1990) PrlA (secY) and PrlG (secE) interact directly and function sequentially during protein translocation in E. coli. Cell 61:833–842
Bieker KL, Phillips GJ, Silhavy T (1990) The sec and prl genes of Escherichia coli. J Bioenerg Biomembr 22:291–310
Brundage L, Hendrick JP, Schiebel E, Driessen AJM, Wickner W (1990) The purified E. coli integral membrane protein secY/E is sufficient for reconstitutions of secA-dependent precursor protein translocation. Cell 62:649–657
Creighton TE (1986) Disulfide bonds as probes of protein folding pathways. Methods Enzymol 131:83–106
Davis BJ (1964) Disc electrophoresis II. Ann N Y Acad Sci 121:405–427
Engels JW, Haas-Lauterbach S (1990) Protein Design am Beispiel des Tendamistats. GIT Fachzeitschrift Labor 9:1037–1044
Engels JW, Koller K-P (1992) Gene expression and secretion of eukaryotic foreign proteins in Streptomyces. In: Murray JAH (ed) Transgenesis. Wiley, New York, pp 31–53
Fitts R, Reuveny Z, Van Amsterdam J, Mulholland J, Botstein D (1987) Substitution of tyrosine for either cysteine in β-lactamase prevents release from membrane during secretion. Proc Natl Acad Sci 84:8540–8543
Hao J, Pazlavova J, Strnadova M, Tichy P, Chaloupka T (1988) Effect of temperature on α-amylase formation and DNA replication in Bacillus subtilis. Folia Microbiol 33:230–233
Hartl F-U, Lecker S, Schiebel E, Hendrick JP, Wickner W (1990) The binding cascade of secB to secA to secY/E mediates preprotein targeting to the E. coli plasma membrane. Cell 63:269–279
Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schrempf H (1985) Genetic manipulation of Streptomyces: a laboratory manual. John Innes Foundation, Norwich
Kline AD, Wüthrich K (1985) The secondary structure of an α-amylase polypeptide inhibitor Tendamistat, from Streptomyces tendae determined in solution by 1H nuclear magnetic resonance. J Mol Biol 183:503
Kline AD, Wüthrich K (1988) Determination of the complete three dimensional structure of an α-amylase inhibitor Tendamistat in aqueous solution by nuclear magnetic resonance and distance geometry. J Mol Biol 204:675
Koller K-P, Engels JW, Uhlmann E (1984) Gene amplification and overproduction of the α-amylase inhibitor (Hoe-467, Tendamistat) in Streptomyces tendae. In: Eds: European Federation of Biotechnology 10–14. Sept. 1984, München. Proceedings of the Third European Congress on Biotechnology, vol 3. Verlag Chemie, Weinheim, pp 273–278
Koller K-P, Riess G, Sauber K, Uhlmann E, Wallmeier H (1989) Recombinant Streptomyces lividans secretes a fusion protein of Tendamistat and proinsulin. Bio/Technology 7:1055–1059
Kramer W, Druta V, Jansen HW, Kramer B, Pflugfelder M, Fritz HJ (1984) The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res 12:9441–9456
Kumamoto CA (1989) Escherichia coli secB protein associated with exported protein precursors in vivo. Proc Natl Acad Sci USA 86:5320–5324
Low C, Parks LW (1987) Sterol phospholipid acyl alterations in Saccharomyces cerevisiae secretion mutants as a function temperature stress. Lipids 22:715–720
Markevicz LJ, Jacques NA (1985) Enhanced secretion of glucosyl-transferase by changes in potassium ion concentrations is accompanied by an altered pattern of membrane fatty acids in Streptococcus salivarius. J Bacteriol 161:989–994
Matsuyama S, Akimaru J, Mizushima S (1990) SecE-Dependent overproduction of secY in Escherichia coli. Evidence for interaction between two components of the secretory machinery. FEBS Lett 269:96–100
Matteucci MD, Caruthers MH (1981) Synthesis of deoxyoligonucleotides on a polymer support. J Am Chem Soc 103:3185–3191
Messing J, Vieira J (1982) A new pair of M13 vectors for selecting either DNA strand of double digest restriction fragments. Gene 19:269
Moore S, Stein WH (1963) Chromatographic determination of amino acids by the use of automatic recording equipment. Methods Enzymol 6:819
Olsson A, Moks T, Uhlen M, Gaal A (1984) Uniformly spaced banding pattern in DNA*** sequencing*** gels by use field strength gradient. J Biochem Biophys Methods 10:83–91
Ornstein L (1964) Disc electrophoresis I. Ann N Y Acad Sci 121:321–349
Paton JC, McMurchie EJ, May KB, Elliot WH (1978) Effect of growth temperature on membrane fatty acids. Composition and susceptibility to cold shock of Bacillus amyloliquefaciens. J Bacteriol 135:754–759
Pflugrath JW, Wiegand G, Huber R (1986) Crystal structure determination, refinement and the molecular model of the α-amylase inhibitor Hoe-467 A. J Mol Biol 189:383–386
Plough M, Jensen AL, Barkholt V (1989) Determination of amino acid compositions and NH2 terminal sequences of peptide electroblotted onto PVDF membranes from Tricine sodium dodecysulphate polyacrylamide gel electrophoresis: application to peptide mapping of human complement component C3. Anal Biochem 181:33–39
Riddles PW, Blakeley RL, Zerner B (1983) The novel use of the Ellman's reagent. Methods Enzymol 91:49–60
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schägger H, Jagow G von (1987) Tricine sodium dodecyl sulphate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Schatz PJ, Beckwith J (1990) Genetic analysis of protein export in Escherichia coli. Annu Rev Genet 24:215–248
Schiebel E, Dreissen AJM, Hartl F-U, Wickner W (1991) Δμh + and ATP function of different stops of catalytic cycle of preprotein translocase. Cell 64:927–939
Schmitt-John T, Engels JW (1992) Promotor constructions for efficient secretion expression in Streptomyces lividans. Appl Microbiol Biotechnol 36:493–498
Tani K, Shiozuka K, Tokuda H, Mizushima S (1989) In vitro analysis of the process of translocation of OmpA across the Escherichia coli cytoplasmic membrane. A translocation intermediate accumulates transiently in the absence of the proton motive force. J Biol Chem 264:18582–18588
Taniyama Y, Yamamoto Y, Nakao M, Kikuchi M, Ikehara M (1988) Role of the disulfide bonds in folding and secretion of human lysozyme in Saccharomyces cerevisiae. Biochem Biophys Res Commun 152:962–967
Vértesy L, Oedin V, Bender R, Zepf K, Nesemann G (1984) Tendamistat (Hoe-467), a tight binding α-amylase inhibitor from Streptomyces tendae 4158. Eur J Biochem 141:505–512
Wickner W, Driessen AJM, Hartl F-U (1991) The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem 60:101–124
Author information
Authors and Affiliations
Additional information
Correspondence to: J. W. Engels
Rights and permissions
About this article
Cite this article
Haas-Lauterbach, S., Scharf, M., Sprunkel, B. et al. High yield fermentation and purification of Tendamistat disulphide analogues secreted by Streptomyces lividans . Appl Microbiol Biotechnol 38, 719–727 (1993). https://doi.org/10.1007/BF00167134
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00167134