Summary
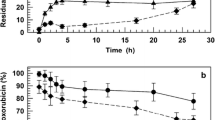
A rat model was used to evaluate the general acute toxicity and the late cardiotoxicity of 4 mg/kg doxorubicin (DOX) given either as free drug or in the form of threeN-(2-hydroxypropyl)methacrylamide (HPMA) copolymer conjugates. In these HPMA copolymers, DOX was covalently bound via peptide linkages that were either non-biodegradable (Gly-Gly) or degradable by lysosomal proteinases (Gly-Phe-Leu-Gly). In addition, one biodegradable conjugate containing galactosamine was used; this residue was targeted to the liver. Over the first 3 weeks after the i.v. administration of free and polymer-bound DOX, all animals showed a transient reduction in body weight. However, the maximal reduction in body weight seen in animals that received polymer-bound DOX (4 mg/kg) was significantly lower than that observed in those that received free DOX (4 mg/kg) or a mixture of the unmodified parent HPMA copolymer and free DOX (4 mg/kg;P<0.01). Throughout the study (20 weeks), deaths related to cardiotoxicity were observed only in animals that received either free DOX or the mixture of HPMA copolymer and free DOX; in these cases, histological investigations revealed marked changes in the heart that were consistent with DOX-induced cardiotoxicity. Sequential measurements of cardiac output in surviving animals that received either free DOX or the mixture of HPMA copolymer and free DOX showed a reduction of ≈30% in function beginning at the 4th week after drug administration. The heart rate in these animals was ≈12% lower than that measured in age-matched control rats (P<0.05). Animals that were given the HPMA copolymer conjugates containing DOX exhibited no significant change in cardiac output throughout the study (P<0.05). In addition, no significant histological change was observed in the hearts of animals that received DOX in the form of HPMA copolymer conjugates and were killed at the end of the study. However, these animals had shown a significant increase in heart rate beginning at 8 weeks after drug administration (P<0.01). This study demonstrates that covalent binding of DOX to HPMA copolymer conjugates via both stable and biodegradable peptidyl linkages considerably reduces both the general acute toxicity and the late cardiotoxicity of DOX in the rat and could offer the potential for improving the therapeutic index in the clinical application of DOX.
Similar content being viewed by others
References
Bertazzoli C, Bellini O, Magrini U, Tosana MG (1979) Quantitative experimental evaluation of Adriamycin cardiotoxicity in the mouse. Cancer Treat Rep 63: 1877
Blum RH (1975) An overview of studies with Adriamycin in the United States. Cancer Chemother Rep 6: 247
Cassidy J, Duncan R, Morrison GJ, Strohalm J, Plocova D, Kopecek J, Kaye SB (1989) Activity ofN-(2-hydroxypropyl)methacrylamide copolymers containing daunomycin against a rat tumour model. Biochem Pharmacol 38: 875
D'Alessandro N, Nicotra C, Crescimanno M, Rausa L (1987) Effects of doxorubicin on mouse heart catalase. Drugs Exp Clin Res 13 (10): 601
Doroshow JH (1988) Role of reactive oxygen production in doxorubicin cardiac toxicity. In: Hacker MP, Lazo JS, Tritton TR (eds) Organ directed toxicities of anticancer drugs. Martinus Nijhoff, Boston, p 31
Duncan R, Cable HC, Rejmanova P, Kopecek J, Lloyd JB (1984) Tyrosinamide residues enhance pinocytic capture ofN-(2-hydroxypropyl)methacrylamide copolymers. Biochim Biophys Acta 799: 1
Duncan R, Cable HC, Lloyd JB, Rejmanova P, Kopecek J (1984) Polymers containing enzymatically degradable bonds: 7. Design of oligopeptide side chains in polyN-(2-hydroxypropyl)methacrylamide copolymers to promote efficient degradation by lysosomal enzymes. Makromol Chem 184: 1997
Duncan R, Kopeckova-Rejmanova P, Strohalm J, Hume I, Cable HC, Pohl J, Lloyd JB, Kopecek J (1987) Anticancer agents coupled toN-(2-hydroxypropyl)methacrylamide copolymers: 1. Evaluation of daunomycin and puromycin conjugates in vitro. Br J Cancer 55: 165
Duncan R, Kopeckova-Rejmanova P, Strohalm J, Hume I, Lloyd JB, Kopecek J (1988) Anticancer agents coupled toN-(2-hydroxypropyl)methacrylamide copolymers: 2. Evaluation of daunomycin conjugates in vivo against L1210 leukaemia. Br J Cancer 57: 147
Duncan R, Hume IC, Kopeckova P, Ulbrich K, Strohalm J, Kopecek J (1989) Anticancer agents coupled toN-(2-hydroxypropyl)methacrylamide copolymers: 3. Evaluation of Adriamycin conjugates against mouse leukaemia L1210 in vivo. J Controlled Release 10: 51
Duncan R, Hume IC, Yardley HJ, Flanagan PA, Ulbrich K, Subr V, Strohalm J (1991) Macromolecular prodrugs for use in cancer chemotherapy: melphalan covalently coupled toN-(2-hydroxypropyl)methacrylamide copolymers. J Controlled Release (in press)
Gabizon A, Shiota R, Papahadjopoulos D (1989) Pharmacokinetics and tissue distribution of doxorubicin encapsulated in stable liposomes with long circulation times. J Natl Cancer Inst 81: 1484
Gebbia N, Leto G, Gagliano M, Tumminelle FM, Rousa L (1985) Lysosomal alterations in heart and liver of mice treated with doxorubicin. Cancer Chemother Pharmacol 15: 26
Green M (1987) Rationale and strategy for prevention of anthracycline cardiotoxicity with the bisdioxopiperazine, ICRF-187. Pathol Biol (Paris) 35: 49
Herman EH, Ferrans VJ, Myers CE, Vleet JF van (1985) Comparison of the effectiveness of (±)-1,2-bis(3,5-dioxopiperazinyl-1-yl) propane (ICRF-187) andN-acetylcysteine in preventing chronic doxorubicin cardiotoxicity in beagles. Cancer Res 45: 276
Hortobagyi GN, Frye D, Buzdar AU, Ewer MS, Fraschini G, Hug V, Ames F, Montague E, Carrasco CH, Mackay B, Benjamin RS (1989) Decreased cardiac toxicity of doxorubicin administered by continuous intravenous infusion in combination chemotherapy for metastatic breast carcinoma. Cancer 63: 37
Kerr DJ, Rogerson A, Morrison GJ, Florence AT, Kage SB (1988) Antitumour activity and pharmacokinetics of niosome encapsulated Adriamycin in monolayer, spheroid and xenograft. Br J Cancer 58: 432
Klugmann S, Bartoli-Klugmann F, Decorti G, Gori D, Silvestri F, Camerini F (1981) Adriamycin experimental antagonistic drugs on ADM-induced cardiomyopathy. Pharmacol Res 13: 769
Lefrak EA, Pitha J, Rosenheim S, Gottlieg JA (1973) A clinicopathologic analysis of Adriamycin cardiotoxicity. Cancer 32: 302
Myers CE (1988) Role for iron in anthracycline action. In: Hacker MP, Lazo JS, Tritton TR (eds) Organ directed toxicities of anticancer drugs. Martinus Nijhoff, Boston, p 17
Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC (1977) Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumour response. Science 197: 165
Neri B, Cini-Neri G, Bandinelli M, Bartalucci S, Ciapio A (1989) Doxorubicin and epirubicin cardiotoxicity: experimental and clinical aspects. Int J Clin Pharmacol Ther Toxicol 27: 217
O'Hare KB, Hume IC, Scarlett L, Duncan R (1989) Evaluation of anticancer agents coupled toN-(2-hydroxypropyl)methacrylamide copolymers. Effect of galactose incorporation on interaction with hepatoma in vitro. Hepatology 10: 207
Rahman A, Schein PS (1988) Use of liposomes in cancer chemotherapy. In: Gregoriadis G (ed) liposomes as drug carriers. John Wiley & Sons, Chichester, p 381
Rejmanova P, Labsky J, Kopecek J (1977) Aminolysis of monomeric and polymericp-nitrophenyl esters of methacryloylated amino acids. Makromol Chem 178: 2159
Rejmanova P, Kopecek J, Duncan R, Lloyd JB (1985) Stability in plasma and serum of lysosomally degradable oligopeptide sequences inN-(2-hydroxypropyl)methacrylamide copolymers. Biomaterials 6: 45
Rihova B, Bilej M, Vetvicka V, Ulbrich K, Strohalm J, Kopecek J, Duncar R (1989) Biocompatibility ofN-(2-hydroxypropyl)methacrylamide copolymers containing Adriamycin. Biomaterials 10: 335
Seymour LW, Ulbrich K, Strohalm J, Kopecek J, Duncan R (1990) Pharmacokinetics of polymer-bound Adriamycin. Biochem Pharmacol 39: 1125
Shapira J, Gotfried M, Lishner M, Ravid M (1990) Reduced cardiotoxicity of doxorubicin by a 6-hour infusion regimen. Cancer 65: 870
Speyer JL, Green MD, Dubin N, Blum RH, Wernz JC, Roses D, Sanger J, Muggia FM (1985) Prospective evaluation of cardiotoxicity during a 6 hour doxorubicin infusion regimen in women with adenocarcinoma of breast. Am J Med 78: 555
Storm G, Van Hoesel QGCM, Groot G de, Kop W, Steerenberg PA, Hillen FC (1989) A comparative study on the antitumour effect, cardiotoxicity and nephrotoxicity of doxorubicin given as a bolus, continuous infusion or entrapped in liposomes in the LON/M WSI rat. Cancer Chemother Pharmacol 24: 341
Strohalm J, Kopecek J (1978) Poly [N-(2-hydroxypropyl)methacrylamide]: IV. Heterogeneous copolymerisation. Angew Makromol Chem 70: 109
Timour Q, Nony P, Lang J, Lakhail M, Triller V, Faucon G (1988) Doxorubicin concentrations in plasma and myocardium and their respective roles in cardiotoxicity. Cardiovasc Drug Ther 1: 559
Trouet A, Jolles G (1984) Targeting of daunomycin by association with DNA or proteins: a review. Semin Oncol 11: 64
Verdun C, Brasseur F, Vranckx H, Couvreur P, Roland M (1990) Tissue distribution of doxorubicin associated with polyisohexylcyanoacrylate nanoparticles. Cancer Chemother Pharmacol 26: 13
Weiss RB, Sarosy G, Clagett-Carr K, Russo M, Leyland-Jones B (1986) Anthracycline analogs: the past, present and future. Cancer Chemother Pharmacol 18: 185
Yeung TK, Simmonds RH, Hopewell JW (1988) Time- and dose-related modifications in cardiac function in rats after single intravenous doses of epirubicin. Radiother Oncol 11: 263
Yeung TK, Simmonds RH, Hopewell JW (1989) A functional assessment of the relative cardiotoxicity of Adriamycin and epirubicin in the rat. Radiother Oncol 15: 275
Yeung TK, Simmonds RH, Hopewell JW (1989) The relative toxicity of intravenous and intraperitoneal doses of epirubicin. Cancer Chemother Pharmacol 24: 211
Zunino F, Pratesi G, Pezzoni G (1987) Increased therapeutic efficacy and reduced toxicity of doxorubicin linked to pyran copolymer via the side chain of the drug. Cancer Treat Rep 71: 367
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yeung, T.K., Hopewell, J.W., Simmonds, R.H. et al. Reduced cardiotoxicity of doxorubicin given in the form ofN-(2-hydroxypropyl) methacrylamide conjugates: an experimental study in the rat. Cancer Chemother. Pharmacol. 29, 105–111 (1991). https://doi.org/10.1007/BF00687318
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00687318