Summary
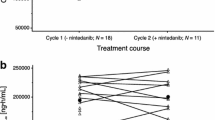
Didemnin B (NSC 325319), a cyclic depsipeptide isolated from a Caribbean tunicate, exhibits potent preclinical antitumor activity. In previous phase I studies, 3.47 mg/m2 was the maximally tolerated dose, with nausea and vomiting being the dose-limiting toxicity. The drug was given in a single bolus infusion over 30 min every 28 days. In the current study, 30 patients presenting with previously treated non-small-cell lung cancer (NSCLC) received 46 courses of the drug at doses ranging from 3.47 to 9.1 mg/m2. Neuromuscular toxicity was dose-limiting. Neusea and vomiting appeared to be correlated with dose levels and were ameliorated by a combination of antiemetics including dexamethasone. Other side effects included a mild rise in hepatic enzymes and an allergic reaction that was preventable by the addition of corticosteroids to the premedication regimen. In all, 2 minor responses were seen among 24 evaluable patients. Because neuromuscular toxicity is dose-limiting, we recommend that routine measurements of creatine kinase and aldolase, a careful neurologic evaluation, and electromyography and muscle biopsy (if indicated) be incorporated into phase II trials. The recommended dose for phase II studies using a single bolus schedule is 6.3 mg/m2, following the premedication of patients with antiemetics.
Similar content being viewed by others
References
Abbruzzese J, Ajani J, Blackburn R, Faintuch J, Patt Y, Levin B (1988) Phase II study of didemnin B in advanced colorectal cancer. Proc Am Assoc Cancer Res 29: A805
Crampton SL, Adams EG, Kuentzel SL, Li LH, Badiner G, Bhuyan BK (1984) Biochemical and cellular effects of didemnins A and B. Cancer Res 44: 1796
Dorr FA, Kuhn JG, Phillips J, Von Hoff DD (1988) Phase I clinical and pharmacokinetic investigation of didemnin B, a cyclic pepsipeptide. Eur J Cancer Clin Oncol 24: 1699
Houssain MB, Van De D, Weinheimer AJ (1988) Crystal and molecular structure of didemnin B, and antiviral and cytotoxic depsipeptide. Proc Natl Acad Sci USA 85: 4118
Jiang TL, Liu RH, Salmon SE (1983) Antitumor activity of didemnin B in the human tumor stem cell assay. Cancer Chemother Pharmacol 11: 1
Li LH, Timmins LG, Wallace TL, Krueger WC, Prairie MD, Im WB (1984) Mechanism of action of didemnin B, a depsipeptide from the sea. Cancer Lett 23: 279
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47: 204
Page JG, Hubbard ST, Kastello MD, Dodds WJ, Grieshaber CK (1985) Effects of two new antineoplastic agents on blood coagulation. Proc Am Assoc Cancer Res 26: 369
Rinehart KL, Gloer JB, Hughes RG, Renis HE, McGovren JP, Swynenberg EB, Stringfellow DA, Kuentzel SL, Li LH (1981) Didemnins: antiviral and antitumor depsipeptides from a Caribbean tunicate. Science 212: 933
Rinehart KL, Shaw PD, Shield LS, Gloer JB, Harbour GC, Koker MES, Samain D, Schwartz RE, Tymiak AA, Weller DL, Carter GT, Munro MHG, Hughes RG, Renis HE, Swynenberg EB, Stringfellow DA, Vavra JJ, Coats JH, Zurenko GE, Kuentzel SL, Li LH, Bakus GJ, Brusca RC, Craft LL, Young DN, Connor JL (1981) Marine natural products as sources of antiviral, antimicrobial and antineoplastic agents. Pure Appl Chem 53: 795
Rossof AH, Rowland K, Khandekar J, Kilton L, Benson AB III, Blough R, Howe H (1989) Phase II trial of didemnin B in previously untreated patients with measurable metastatic colorectal carcinoma. Proc Am Soc Clin Oncol 8: A439
Stewart JA, Tong WP, Hartwhorn JN, McCormack JJ (1986) Phase I evaluation of didemnin B (NSC 325319). Proc Am Soc Clin Oncol 5: A128
Tong WP, Webster LK, Hartshorn JN, Stewart JA, McCormack JJ (1986) Chromographic assay for didemnin B and application to pharmacological studies. Proc Am Assoc Cancer Res 27: A281
Author information
Authors and Affiliations
Additional information
This work was supported in part by grant NO1CM57 739 from the National Cancer Institute. The senior author (D. M. S.) is a recipient of the American Cancer Society Clinical Oncology Career Development Award
Rights and permissions
About this article
Cite this article
Shin, D.M., Holoye, P.Y., Murphy, W.K. et al. Phase I/II clinical trial of didemnin B in non-small-cell lung cancer: neuromuscular toxicity is dose-limiting. Cancer Chemother. Pharmacol. 29, 145–149 (1991). https://doi.org/10.1007/BF00687325
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00687325