Abstract
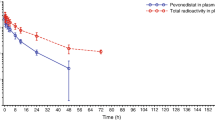
NK 611 is a new podophyllotoxin derivative in which a dimethyl amino group replaces a hydroxyl group at the sugar moiety of etoposide. This results in profound physico-chemical differences: NK 611 is much less hydrophobic than etoposide. Preclinical studies have shown that NK 611 is advantageous in terms of bioavailability and of the potency of its anticancer activity. A clinical phase I study was performed in cancer patients within the framework of the AIO. Additionally, its pharmacokinetics and pharmacodynamics were investigated. NK 611 was given to 26 patients at doses ranging from 60 to 140 mg/m2 [maximum tolerated dose (MTD) 120 mg/m2] in a 30-min infusion. Plasma and urine samples were collected from 25 patients and analyzed using a validated high-performance liquid chromatography (HPLC) assay procedure. The concentration versus time curve of total NK 611 in plasma samples was best described by a three-compartment model. The overall median pharmacokinetic values were as follows (ranges are given in parantheses): mean residence time (MRT) 16.5 (5.4– 42.3)h, terminal half-life 14.0 (8.2–30.5)h, volume of distribution at steady state (Vss) 11.4 (7.9–18.1) l/m2, and plasma clearance (Clp) 15.1 (3.6–36.4) ml min-1 m -2. The total systemic drug exposure, represented by the area under the curve (AUC), varied between 53.4 and 532.0 μg ml-1 h. The mean AUC (±SD) increased with the dose from 78.7±3.7 μg ml-1 h at 60 mg/m2 up to 202.8±157.2 μg ml-1 h at 120 mg/m2. The mean urinary excretion (UE) fraction of unchanged drug at 48 h after the end of the infusion varied between 3.0% and 25.8% of the total dose delivered. Analysis of ultrafiltrate samples showed a protein binding of approx. 99%. The percentage reduction in white blood cells (WBC) and neutrophils (ANC) correlated with the dose, AUC, and AUCfree. The best relationship between the percentage of reduction in ANC and a pharmacokinetic parameter (AUC) took a nonlinear Hill-type form. The laboratory parameter for kidney or liver function did not correlate with the AUC. The variation of pharmacokinetic parameters within each dose level was profound. The reason for this pharmacological behavior remains unclear and should be investigated in further studies.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 8 May 1995/Accepted: 27 October 1995
Rights and permissions
About this article
Cite this article
Mross, K., Hüttmann, A., Herbst, K. et al. Pharmacokinetics and pharmacodynamics of the new podophyllotoxin derivative NK 611 A study by the AIO groups PHASE-I and APOH. Cancer Chemother Pharmacol 38, 217–224 (1996). https://doi.org/10.1007/s002800050474
Issue Date:
DOI: https://doi.org/10.1007/s002800050474