ABSTRACT
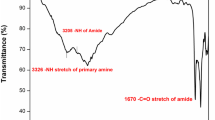
: We have investigated a novel monomer having two pendent phenyl imido groups for preparing new cycloaliphatic-aromatic polyamides. Novel polyamides were synthesized by direct polycondensation reaction of N,N'-diphenyl-2,3,5,6-diimido cyclohexane-1,4-dicarboxylic acid(PICA) and various aromatic diamines such as p-phenylene diamine, 4,4'-oxydianiline and 4,4'-methylene dianiline. A direct polycondensation was carried out by a Yamazaki method which is typical of using triphenyl phosphite, lithium chloride, and pyridine. Inherent viscosity of these resulting polyamides are ranged 0.20 ∼ 0.45 dl/g. A transparent flexible and tough film was casted. The glass transition temperature of the polyamide from the PICA and 4,4'-oxydianiline is 147 °C. The decomposition temperature of these polyamides are ranged from 350 ∼ 360 °C and the ash contents of them orders MDA > p-PDA > ODA according to kinds of the using diamines. And the solubilities of these polyamides are good in aprotic solvents such as DMAc, NMP, DMF.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 26 July 1999/Revised version: 14 September 1999/Accepted: 28 September 1999
Rights and permissions
About this article
Cite this article
Park, S., Lee, J. & Suh, D. Synthesis and the properties of novel cycloaliphatic-aromatic polyamides having pendent N,N'-diphenyl imido groups. Polymer Bulletin 43, 311–318 (1999). https://doi.org/10.1007/s002890050616
Issue Date:
DOI: https://doi.org/10.1007/s002890050616