Abstract
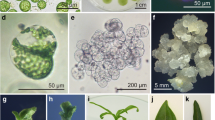
Viable protoplasts of Vigna sublobata L. were isolated enzymatically from hypocotyls of axenic seedlings. Protoplast yields were dependent upon seedling age, with maximum yields (2.25 ± 0.35 × 106 g fwt−1) from seedlings aged 6 d. Protoplasts regenerated cell walls and underwent sustained divisions when cultured in either agarose-solidified or liquid K8P medium. The plating density affected the division frequency and plating efficiency; the division frequency (68 ±0 6.0%) was maximum at 4.0 × 104 ml−1 while plating efficiency was maximum (1.3 ± 0.1%) at 5.0 × 104 ml−1. Dividing protoplasts developed into microcalli, which produced glossy green compact nodular calli on transfer to 8.0 gl−1 w/v agar-solidified medium containing MS salts, B5 organic components, 30 g l−1 sucrose, NAA (0.2–0.5 mg l−1), zeatin riboside (0.5–2.0 mg l−1) and GA3 (0.5–1.0 mg l−1). These calli, after sub-culture on the same medium, produced shoot buds which underwent elongation following transfer of tissues to 6.0 g l−1 agar-solidified B5 medium containing 30g l−1 sucrose, IBA (0.01 mg l−1) and BAP (1.0 mg l−1). Elongated shoots developed roots after transfer to 8.0g l−1 agar-solidified, hormone-free MS medium with 30 g l−1 sucrose.
Similar content being viewed by others
Abbreviations
- IAA:
-
indole-3-acetic acid
- IBA:
-
indole-3-butyric acid
- BAP:
-
6-benzyladenine or benzylaminopurine
- B5:
-
medium after Gamborg et al (1968)
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- GA3 :
-
gibberellic acid
- 2,i-P:
-
6-(γ-γ-dimethylallylamino) purine
- MS:
-
medium after Murashige and Skoog (1962)
- NAA:
-
1-naphthaleneacetic acid
References
Bhadra SK, Hammatt N, Davey MR (1990) Callus induction from seedling protoplasts of Vigna gracilis and V. trilobata. SABRAO J. 22: 25–33.
Bhadra SK, Hammatt N, Davey MR (1991) Tissue and protoplast culture of rice bean [Vigna umbellata (Thunb.) Ohwi and Ohashi]. Trop Agric 68: 344–348.
Bhargava S, Chandra N (1983) In vitro differentiation in callus cultures of moth bean, Vigna aconitifolia (Jacq) Marechal. Plant Cell Rep 2: 47–50.
Frearson EM, Power JB, Cocking EC (1973). The isolation, culture and regeneration of Petunia leaf protoplasts. Dev Biol 33: 130–137.
Gamborg OL, Miller RA, Ojima K (1968). Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158.
Gill R, Eapen S (1986). Plant regeneration from hypocotyl protoplasts of moth bean (Vigna aconitifolia). Curr Sci 55: 100–102.
Gill R, Eapen S, Rao PS (1986). Tissue culture studies in moth bean — factors influencing plant regeneration from seedling expiants of different cultivars. Proc Indian Acad Sci (Plant Sci) 96: 55–61.
Gill R, Eapen S, Rao PS (1987). Callus induction from protoplasts of V. unguiculata, V. sublobata and V. mungo. Theor Appl Genet 74: 100–103.
Hammatt N, Davey MR (1988). Isolation and culture of soybean hypocotyl protoplasts. In Vitro Cell Dev Biol 24: 601–604.
Hammatt N, Kim H-I, Davey MR, Nelson RS, Cocking EC (1987). Plant regeneration from cotyledon protoplasts of Glycine canescens and G. clandestina. Plant Sci 48: 129–135.
Kao KN (1977). Chromosomal behaviour of somatic hybrids of soybean — Nicotiana glauca. Molec Gen Genet 50: 225–230.
Mukhopadhyay A, Bhojwani SS (1978). Shoot bud differentiation in tissue cultures of leguminous plants. Z Pflanzenphysiol 88: 263–268.
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497.
Wei Z, Xu Z (1988). Plant regeneration from protoplasts of soybean (Glycine max L.). Plant Cell Rep 7: 348–351.
Wilson VM, Haq N, Evans PK (1985). Protoplast isolation, culture and regeneration in the winged bean, Psophocarpus tetragonolobus (L.) DC. Plant Sci 41: 61–68.
Xu ZH, Yang LJ, Wei ZM, Gao MX (1984). Plant regeneration in tissue culture of four leguminous species. Acta Biol Sinica 17: 483–486.
Author information
Authors and Affiliations
Additional information
Communicated by K. Glimelius
Rights and permissions
About this article
Cite this article
Bhadra, S.K., Hammatt, N., Power, J.B. et al. A reproducible procedure for plant regeneration from seedling hypocotyl protoplasts of Vigna sublobata L.. Plant Cell Reports 14, 175–179 (1994). https://doi.org/10.1007/BF00233785
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00233785