Summary
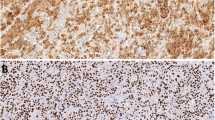
Routinely processed parathyroid tissues from 26 cases with primary hyperparathyroidism (19 adenomas, 7 multiglandular hyperplasia) and 8 normal human parathyroid glands were investigated with antibodies against chromogranin A and B and parathyroid hormone (PTH). Normal parathyroids were immunohistochemically positive for PTH and chromogranin A but negative for chromogranin B. Hyperplastic glands showed a focal staining for PTH and chromogranin A without correlation of the staining pattern on serial sections. Adenomas were either uniformly positive for both PTH and chromogranin A or showed a staining pattern similar to that seen in hyperplastic glands. Focal chromogranin B positivity (less than 10% of cells) was found in 3 cases (1 hyperplastic gland and 2 cases of parathyroid adenoma with an immunohistochemical staining pattern similar to hyperplastic glands). Our immunohistochemical results may support previously published findings that most parathyroid adenomas are monoclonal neoplasms whereas hyperplastic glands are of polyclonal origin.
Similar content being viewed by others
References
Andrew SM, Jasani B (1987) An improved method for the inhibition of endogenous peroxidase non-deleterious to lymphocyte surface markers. Application to immunoperoxidase studies on eosinophil-rich tissue preparations. Histochem J 19:426–430
Arnold A, Staunton CE, Kim HG, Gaz RD, Kronenberg HM (1988) Monoclonality and abnormal parathyroid hormone genes in parathyroid adenomas. N Engl J Med 318:658–662
Cohn DV, Zangerle R, Fischer-Colbrie R, Chu LLH, Elting JJ, Hamilton JW, Winkler H (1982) Similarity of secretory protein I from parathyroid gland to chromogranin A from adrenal medulla. Proc Natl Acad Sci USA 79:6056–6059
Efendic S, Tatemoto K, Mutt V, Quan C, Chang D, Östenson CG (1987) Pancreastatin and islet cell release. Proc Natl Acad Sci USA 84:7257–7261
Fischer-Colbrie R, Frischenschlager I (1985) Immunological characterization of secretory proteins of chromaffin granules: chromogranins A, chromogranins B and enkephalin-containing peptides. J Neurochem 44:1854–1858
Fischer-Colbrie R, Schober M (1987) Isolation and characterization of chromogranins A, B, and C from bovine chromaffin granules and rat pheochromocytoma. J Neurochem 48:262–265
Fischer-Colbrie R, Iacangelo A, Eiden LE (1988) Neural and humoral factors separately regulate neuropeptide Y, enkephalin and chromogranin A and B mRNA levels in rat adrenal medulla. Proc Natl Acad Sci USA 85:3240–3244
Ghandur-Mnaymneh L, Kimura N (1984) The parathyroid adenoma. A histopathologic definition with a study of 172 cases of primary hyperparathyroidism. Am J Pathol 115:70–83
Hagn C, Schmid KW, Fischer-Colbrie R, Winkler H (1986) Chromogranin A, B, and C in human adrenal medulla and endocrine tissues. Lab Invest 55:405–411
Hearn SA (1987) Electron microscopic localization of chromogranin A in osmium-fixed neuroendocrine cells with a protein A-gold technique. J Histochem Cytochem 35:795–801
Helle KB (1966) Some chemical and physical properties of the soluble protein fraction of bovine adrenal chromaffin granules. Mol Pharmacol 2:298–310
Hittmair A, Schmid KW (1989) Inhibition of endogenous peroxidase for the immunocytochemical demonstration of intermediate filament proteins (IFP). J Immunol Methods 116:199–205
Iacangelo AL, Fischer-Colbrie R, Koller KJ, Brownstein MJ, Eiden LE (1988) The sequence of porcine chromogranin A messenger RNA demonstrates that chromogranin A can serve as the precursor for the biologically active hormone, pancreastatin. J Endocrinol 122:2339–2343
Lloyd RV, Wilson BS (1983) Specific endocrine tissue marker defined by a monoclonal antibody. Science 222:628–630
Lloyd RV, Cano M, Rosa P, Hille A, Huttner WB (1988) Distribution of chromogranin A and secretogranin I (chromogranin B) in neuroendocrine cells and tumors. Am J Pathol 130:296–304
Lloyd RV, Iacangelo A, Eiden LE, Cano M, Jin L, Grimes M (1989) Chromogranin A and B messenger ribonucleic acids in pituitary and other normal and neoplastic human endocrine tissues. Lab Invest 60:548–556
MacGregor RR, Hinton DA, Ridgeway RD (1988) Effects of Ca on synthesis and secretion of PTH and secretory protein I. Am J Physiol 255 [Suppl]: 299–305
Mendelsohn G (1988) Pathology of the parathyroid glands. In: Mendelsohn G (ed) Diagnosis and pathology of endocrine diseases. Lippincott, Philadelphia, pp 139–177
Nanes MS, O'Connor DT, Marx SJ (1989) Plasma chromogranin-A in primary hyperparathyroidism. J Clin Endocrinol Metab 69:950–955
O'Connor DT, Burton D, Deftos LJ (1983) Immunoreactive human chromogranin A in diverse polypeptide hormone producing human tumors and normal endocrine tissues. J Clin Endocrinol Metab 57:1084–1086
Oka T, Yoshioka T, Shrestha GR, Koide T, Sonoda T, Hosokawa S, Onoe K, Sakurai M (1988) Immunohistochemical study of nodular hyperplastic parathyroid glands in patients with secondary hyperparathyroidism. Virchows Arch [A] 413:53–60
Rindi G, Buffa R, Sessa F, Tortora O, Solcia E (1986) Chromogranin A, B and C immunoreactivities of mammalian endocrine cells. Distribution, distinction from co-stored hormones/prohormones and relationship with the argyrophil component of secretory granules. Histochemistry 85:19–28
Rosa P, Hille A, Lee RW, Zanini A, DeCamilli P, Huttner WB (1985) Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol 101:1999–2011
Schmid KW, Fischer-Colbrie R, Hagn C, Jasani B, Williams ED, Winkler H (1987) Chromogranin A and B and secretogranin II in medullary carcinomas of the thyroid. Am J Surg Pathol 11:551–556
Schmid KW, Weiler R, Xu RW, Hogue-Angeletti R, Fischer-Colbrie R, Winkler H (1989) An immunological study on chromogranin A and B in human endocrine and nervous tissues. Histochem J 21:365–373
Schober M, Fischer-Colbrie R, Schmid KW, Bussolati G, O'Connor DT, Winkler H (1987) Comparison of chromogranins A, B, and secretogranin II in human adrenal medulla and pheochromocytoma. Lab Invest 57:385–391
Sekiya K, Ghatei MA, Salahuddin MJ, Bishop AE, Hamid QA, Ibayashi H, Polak JM, Bloom SR (1989) Production of GAWK (chromogranin-B 420-493)-like immunoreactivity by endocrine tumors and its possible diagnostic value. J Clin Invest 83:1834–1842
Simon JP, Bader MF, Aunis D (1988) Secretion from chromaffin cells is controlled by chromogranin A-derived peptides. Proc Natl Acad Sci USA 85:1712–1716
Smith AD, Winkler H (1967) Purification and properties of an acidic protein from chromaffin granules of bovine adrenal medulla. Biochem J 103:483–492
Smith WD, Kirshner N (1967) A specific soluble protein from the chatecholamin storage vesicles of bovine adrenal medulla. Mol Pharmacol 3:52–62
Weiler R, Feichtinger H, Schmid KW, Fischer-Colbrie R, Grimelius L, Cedermark B, Papotti M, Bussolati G, Winkler H (1987) Chromogranin A and B and secretogranin II in bronchial and intestinal carcinoids. Virchows Arch [A] 412:103–109
Weiler R, Fischer-Colbrie R, Schmid KW, Feichtinger H, Bussolati G, Grimelius L, Krisch K, Kerl H, O'Connor D, Winkler H (1988) Immunological studies on the occurrence and properties of chromogranin A and B and secretogranin II in endocrine tumors. Am J Surg Pathol 12:877–884
Wiedenmann B, Huttner WB (1989) Synaptophysin and chromogranins/secretogranins — widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch [B] 58:95–121
Wilson BS, Lloyd RV (1984) Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol 115:458–468
Winkler H, Apps DK, Fischer-Colbrie R (1986) The molecular function of adrenal chromaffin granules: established facts and unresolved topics. Neuroscience 18:261–290
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmid, K.W., Hittmair, A., Ladurner, D. et al. Chromogranin A and B in parathyroid tissue of cases of primary hyperparathyroidism: an immunohistochemical study. Vichows Archiv A Pathol Anat 418, 295–299 (1991). https://doi.org/10.1007/BF01600157
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01600157