Summary
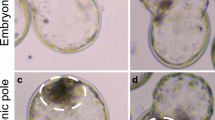
We have used immunohistochemical techniques to study laminin in quail blastoderms milked from the oviduct and the distribution of laminin in laid chicken and quail blastoderms. Laminin is a constituent of the basement membrane in both chicken and quail blastoderms. It is found at the ventral side of the upper layer cells. Laminin is first observed under individual upper layer cells in prelaid quail blastoderms 15 h post-ovulation, but is absent at the ingression site of endophyll cells. The presence of a continuous laminin layer coincides with the epithelialization of the epiblast after 5–10 h incubation. The laminin layer is discontinuous at the primitive streak and at Hensen's node. It is thinner and partly discontinuous at the median part of the neural plate. By induction, either of an ectopic primitive streak or a neural plate, we have demonstrated, using the chicken-quail nucleolar marker technique, that at these sites the laminin layer is interrupted. A laminin layer might confer rigidity onto the epiblast, whereas disruption of a laminin layer seems to be correlated with ingression of cells or bending of the neural plate.
Similar content being viewed by others
References
Albrechtsen R, Nielsen M, Wewer U, Engvall E, Ruoslahti E (1981) Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res 41:5076–5081
De Bruyne GK, Bracke ME, Plessers L, Mareel MM (1988) Invasiveness in vitro of mixed aggregates composed of two human mammary cell lines MCF-7 and HBL-100. Invasion Metastasis 8:253–265
Duband J-L, Thiery JP (1982) Appearance and distribution of fibronectin during chick embryo gastrulation and neurulation. Dev Biol 94:337–350
Duband J-L, Thiery JP (1987) Distribution of laminin and collagens during avian neural crest development. Development 101:461–478
Eyal-Giladi H, Kochav S (1976) From cleavage to primitive streak formation: A complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev Biol 49:321–337
Hamburger V, Hamilton H (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Kleinman HK, Cannon FB, Laurie GW, Hassell JR, Aumailley M, Terranova VP, Martin GR, DuBois-Dalcq M (1985) Biological activities of laminin. J Cell Biochem 27:317–325
Le Douarin NM (1973) A Feulgen-positive nucleolus. Exp Cell Res 77:459–468
Leivo I, Vaheri A, Timpl R, Wartiovaara J (1980) Appearance and distribution of collagens and laminin in the early mouse embryo. Dev Biol 76:100–114
Lison L (1960) Histochimie et cytochimie animales, principes et méthodes, vol II. 3 edn. Gauthier-Villars, Paris, pp 398–842
Low FN (1967) Developing boundary (basement) membranes in the chick embryo. Anat Rec 159:231–238
Martin GR, Timpl R (1987) Laminin and other basement membrane components. Ann Rev Cell Biol 3:57–85
Merbach H (1935) Beobachtungen an der Keimscheibe des Hühnchens vor dem Erscheinen des Primitivstreifens. Z Anat Entwicklungsgesch 104:635–652
Mitrani E (1982) Primitive streak-forming cells of the chick invaginate through a basement membrane. Wilhelm Roux's Arch 191:320–324
Romeis B (1968) Mikroskopische Technik, 16 edn, R. Oldenbourg Verlag, München Wien, 757
Sanders EJ, Prasad S (1986) Epithelial and basement membrane responses to chick embryo primitive streak grafts. Cell Differ 18:233–242
Smith JL, Schoenwolf GC (1987) Cell cycle and neuroepithelial cell shape during bending of the chick neural plate. Anat Rec 218:196–206
Timpl R, Rohde H, Robey P, Rennard S, Foidart J-M, Martin G (1979) Laminin-a glycoprotein from basement membranes. J Biol Chem 254 19:9933–9937
Vakaet L (1970) Cinephotomicrographic investigations of gastrulation in the chick blastoderm. Arch Biol (Liège) 81:387–426
Vakaet L (1984) The initiation of gastrular ingression in the chick blastoderm. Am Zool 24:555–562
Vakaet L, Vanroelen Chr, Andries L (1980) An embryological model of non-malignant invasion or ingression. In: De Brabander M, is De Brabander M, Mareel M, De Ridder L (eds) Cell movement and neoplasia. Pergamon Press, Oxford New York, pp 65–75
Woodard AE, Mather FB (1964) The timing of ovulation, movement of the ovum through the oviduct, pigmentation and shell deposition in the Japanese quail (Coturnix coturnix japonica). Poultry Sci 43(6):1427–1432
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bortier, H., De Bruyne, G., Espeel, M. et al. Immunohistochemistry of laminin in early chicken and quail blastoderms. Anat Embryol 180, 65–69 (1989). https://doi.org/10.1007/BF00321901
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00321901