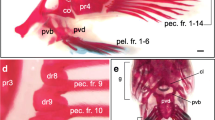
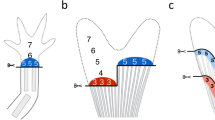
Summary
The pectoral fin of blennies is differentiated into a dorsal field and a ventral hook field. A histogenetic analysis of the regenerating pectoral fin was related to two questions. First, are histological specializations of the hook field responsible for the impairment of the regenerative capacity of pectoral fins of blennies? Second, can analysis of the temporal sequence of histogenetic events be used to make testable predictions about the tissue interactions required to re-establish the adult pattern? Regeneration of pectoral fins was examined in Salaria pavo (Blenniidae, Teleostei). Approximately 80% of the length of the fin rays was amputated. Fin ray stumps were evaluated 7,14, 24, 48 and 72 h after amputation, regenerates 4, 5, and 6 days after amputation and at length of about 30%, 50% and 60% regeneration of the original fin length. The regeneration process is subdivided into four stages: wound healing, blastema formation, fin ray formation and distal outgrowth and differentiation of hook characters. Analysis of the early events of regeneration, wound healing, blastema formation and distal outgrowth, yielded no profound differences from those of conventional fins in general. Impairment of regenerative capacity becomes manifested before histological differentiation of hook characters, and it is thus unlikely that their presence is the proximate cause of heteromorphic regeneration. The sequence in which the anatomical specializations characteristic of fin hooks (lepidotrichal cord, cuticle, fin web regression) appear was variable. Detailed analysis of older regenerates revealed a more regular pattern. In the first phase the characters appear to be largely independently organized, while they become locally correlated later. It is concluded that the anatomical differentiation passes through two stages, initiation of anatomical differentiation, and then mutual adjustment of character expression leading to spatially correlated expression of the lepidotrichal cord, the cuticle and the fin web regression.
Similar content being viewed by others
Abbreviations
- ac :
-
actinotrichia
- ahr :
-
anterior hemi ray
- cu :
-
cuticle
- dac :
-
dense association of cells
- phr :
-
posterior hemi ray
- fw :
-
fin web
- fb :
-
fibroblasts
- hr :
-
hemi ray
- hsb :
-
hemi segment border
- iwe :
-
inner layer of the wound epithelium
- ilt :
-
intralepidotrichal tissue
- lac :
-
loose association of cells
- lc :
-
lepidotrichal cord
- mc :
-
mesenchymal collumn
- mwe :
-
middle layer of the wound epithelium
- pmc :
-
posterior mc
- scb :
-
scleroblasts
- sv :
-
serous vesicles
References
Baguná J, Sálo E, Collet J, Auladell MC, Bibas M (1988) Cellular, molecular and genetic approaches to regeneration and pattern formation in planarians. Prog Zool 16:65–78
Baguná J, Sálo E, Auladell C (1989) Regeneration and pattern formation in planarians. III. Evidence that neoblasts are totipotent stem cells and the source of blastema cells. Development 107:77–86
Banz E (1935) Zur Morphologie und Histologie der Flossenregeneration bei Knochenfischen unter besonderer Berücksichtigung von Misgurnus fossilis L. Zool Anz 112:209–230
Becerra J, Montes GS, Bexiga SRR, Junqueira lcU (1983) Structure of the tail fin in teleosts. Cell Tissue Res 230:127–137
Beigel C (1910) Zur Regeneration des Kiemendeckels und der Flossen der Teleostier. Arch Entwicklungsmech Org 34:543–547
Berill NJ (1952) Regeneration and budding in worms. Biol Rev 27:401–438
Birnie JH (1934) Regeneration of the tail fins of Fundulus embryos. Biol Bull 66:316–325
Blanc M (1949) Etude histologique de la régéneration des nageoires chez quelques poisson Téléostéens. Arch Anat Microsc Morp Exp 38:52–64
Bogacki K (1906) Experimentelle Flossenregeneration bei europäischen Süßwasserfischen. Arch Entwicklungsmech Org 22:18–21
Brandstätter R, Misof BY, Pázmándi C, Wagner GP (1990) Microanatomy of the pectoral fin in blennies (Blenniini, Teleostei). J Fish Biol 37:729–743
Brockes JP (1989) Retinoids, Homeobox genes, and limb morphogenesis. Neuron 2:1285–1294
Cummings SG, Bode HR (1984) Head regeneration and polarity reversal in Hydra attenuata can occur in absence of DNA synthesis. Wilhelm Roux Arch Dev Biol 194:79–86
Del Cerro M, et al (1980) Stevenel's blue, an excellent stain for microscopical study of platic embedded tissues. Microsc Acta 83:117–121
Duncker G (1905) Über Regeneration des Schwanzendes bei Syngnathiden. Roux's Arch Entwicklungsmech Org 20:30–37
Eskin IA (1928) Zur Frage nach der Regeneration der Flossen bei den Fischen. Zool Anz 79:289–304
Géraudie J (1977) Initiation of the actinotrichial development in the early fin bud of the fish, Salmo. J Morphol 151:353–362
Géraudie J, Landis WJ (1982) The fine structure of the developing pelvic fin dermal skeleton in the trout Salmo gairdneri. Am J Anat 163:141–156
Géraudie J, Singer M (1977) Relation between nerve fiber number and pectoral fin regeneration in teleost. J Exp Zool 199:1–8
Géraudie J, Singer M (1985) Necessity of an adequate nerve supply for regeneration of the amputated pectoral fin in the teleost Fundulus. J Exp Zool 234:367–374
Goss RJ (1969) Principles of regeneration. Academic Press, New York London
Goss RJ, Stagg MW (1957) The regeneration of fins and fin rays in Fundulus heteroclitus. J Exp Zool 136:487–508
Haas HJ (1962) Studies on mechanisms of joint and bone formation in the skeleton rays of fish fins. Dev Biol 5:1–34
Hinchliffe JR, Griffiths PJ (1986) Vital staining for cell death in chick limb buds: a histochemical technique in the analysis of control of limb development. Acta Histochem [Suppl] XXXII: 159–164
Ingham PW (1988) The molecular genetics of embryonic pattern formation in Drosophila. Nature 335:25–34
Jacobson AG, Sater AS (1988) Features of embryonic induction. Development 104:341–359
Kauffman SJ (1969) Metabolic stability and epigenesis in randomly constructed genetic nets. J Theor Biol 22:3
Kemp NE, Park JH (1970) Regeneration of lepidotrichia and actinotrichia in the tail fin of the teleost Tilapia mossambica. Dev Biol 22:321–342
Kemp NE, Park JH, McWilliams AK, Auerbach SE, Bozdech MJ (1968) Fine structure of lepidotrichia and actinotrichia in regenerating tail fins of teleosts. Anat Rec 160:374
Laming PR (1982) Behavioral, physiological and morphological adaptations of the shanny (Blennius pholis) to the intertidal habitat. J Mar Biol Assoc UK 62:329–338
Lanzing WJR (1976) The fine structure of fins and fin rays of Tilapia mossambica (Peters). Cell Tissue Res 173:349–356
Lumsden A (1990) The cellular basis of segmentation in the developing hindbrain. Trends Neurosci 13:328–335
Lumsden A, Keynes R (1989) Segmental patterns of neuronal development in the chick hindbrain. Nature 337:424–428
María-Beffa M, Carmona MG, Becerra J (1989) Elastoidin turnover durin tail fin regeneration in teleosts. Anat Embryol 180:465–470
Mittal AK, Banerjee TK (1979) Keratinization versus mucous secretion in fish epidermis. In: Spearman RIC, Riley PA (eds) The skin of vertebrates. Symp Linn Soc 7. Academic Press, London
Montes GS, Becerra J, Toledo OMS, Gordilho MA, Junqueira LCU (1982) Fine structure and histochemistry of the tail fin ray in the teleosts. Histochemistry 75:363–376
Morgan TH (1900) Regeneration in teleosts. Arch Entwicklungsmech Org X: 120–131
Morgan TH (1902) Further experiments on the regeneration of the tail of fishes. Arch Entwicklungsmech Org XIV: 539–561
Morrill CV (1906) Regeneration of certain structures in Fundulus heteroclitus. Biol Bull 12:11–20
Nabrit SM (1929) The role of the fin rays in the regeneration in the tail fins of fishes (in Fundulus and Goldfish). Biol Bull LVI: 235–266
Nabrit SM (1931) The role of the basal plate of the tail in regeneration in the tail fins of fishes (Fundulus and Carassius). Biol Bull 60:60–63
Nusbaum J, Sidoriak S (1900) Beiträge zur Kenntnis der Regenerationsvorgänge nach künstlichen Verletzungen bei älteren Bachforellenembryonen (Salmo fario L.) Arch Entwicklungsmech Org 10:645–684
Okada YK (1943) Regeneration of the tail in fish. Annotationes Zoologicae Japonenses 22:59–69
Pázmándi CH (1991) The pectoral anatomy of Salaria fluviatilis. Thesis, University of Vienna
Saló E, Baguná J (1989) Regeneration and pattern formation in planarians. II. Local origin and the role of cell movements in blastema formation. Development 107:69–76
Santamaria JA, Becerra J (1991) Tail fin regeneration in teleosts: cell-extracellular matrix interaction in blastemal differentiation. J Anat 176:9–21
Sauter V (1934) Regeneration and Transplantation bei erwachsenen Fischen. Wilhelm Roux' Arch Entwicklungsmech Org 132:1–41
Scott GG (1909) Regeneration in Fundulus and its relation to the size of the fish. Biol Bull 17:343–353
Stocum DL (1991) Limb regeneration: a call to arms (and legs). Cell 67:5–8
Wagner GP (1989) The origin of morphological characters and the biological basis of homology. Evolution 43:1157–1171
Wagner GP, Almeder M (1991) Epigenetic control of fin web shape in Lipophrys canevae. Experientia 47:737–738
Wagner GP, Misof BY (1992) Evolutionary modification of regenerative capability in vertebrates: a comparative study on teleost pectoral fin regeneration. J Exp Zool 261:62–78
Wickler W (1960) Die Stammesgeschichte typischer Bewegungsformen der Fisch-Brustflosse. Z Tierpsychol 17:31–66
Wilkinson DG, Krumlauf R (1990) Molecular approaches to the segmentation of the hindbrain. Trends Neurosci 13:335–339
Whitear M (1986) Epidermis. In: Bereiter-Hahn J (ed) Biology of the Integument, vol 2. Springer, Berlin Heidelberg New York, pp 9–64
Whitear M, Mittal AK (1984) Surface secretion of the skin of Blennius (Lipophrys) pholis L. J Fish Biol 25:317–331
Wolpert E (1989) Positional information revisited. Development [Suppl] 3–12
Wood A (1982) Early pectoral fin development and morphogenesis of the apical ectodermal ridge in the killifish, Aphyosemion scheeli. Anat Rec 204:349–356
Wood A, Thorogood P (1984) An analysis of in vivo cell migration during teleost fin morphogenesis. J Cell Sci 66:205–222
Wood A, Thorogood P (1987) An ultrastructural and morphometric analysis of an in vivo contact guidance system. Development 101:363–381
Zander CD (1970) Zur Morphologie der Flossen von Blenniidae (Pisces) des Mittelmeeres. J Ichthyol 22:93–96
Zander CD (1972) Beziehung zwischen Körperbau und Eebensweise bei Blenniidae (Pisces) aus dem Roten Meer. 2. Bau der Flossen und ihrer Muskulatur. Z Morphol Ökol Tiere 71:299–327
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Misof, B.Y., Wagner, G.P. Regeneration in Salaria pavo (Blenniidae, Teleostei). Anat Embryol 186, 153–165 (1992). https://doi.org/10.1007/BF00174953
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00174953