Summary
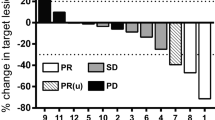
In order to develop a more dose-intensive induction regimen for the treatment of far-advanced testicular tumours, the German Cooperative Group for Testicular Tumours started a dose-escalation trial of cisplatin, etoposide and ifosfamide. At the first dose level 18 patients with advanced testicular cancer (Indiana University classification) received cisplatin 25 mg/m2, etoposide 120–150 mg/m2 and ifosfamide 1.2 g/m2 for 5 days every 3 weeks. Of these, 13 patients (72%) became tumour-free, 2 achieved a stable, marker-negative partial remission, 2 had progressive disease and 1 patient died ofClostridium sepsis. The main toxicity was myelosuppression with a white blood cell nadir of 900/μl and a thrombocyte nadir of 47000/μl. Granulocytopenic fever occurred in 43% of all cycles. At the second dose level 15 patients received cisplatin 30 mg/m2, etoposide 150 mg/m2 and ifosfamide 1.6 g/m2 five times every 3 weeks together with s.c. recombinant granulocyte/macrophage-colony-stimulating factor (GM-CSF) 10 μg/kg on days 6–15. Acute toxicity was severe with a white blood cell nadir of 300/μl and thrombocyte nadir of 11 000/μl. The duration of the thrombocytopenia increased with cycle number; 63% of all cycles were associated with granulocytopenic fever and in 83% platelet transfusions were required. One patient died from acute renal failure andAspergillus sepsis; 3 patients experienced adverse reactions to GM-CSF, requiring omission of this drugs in 2; 33% had grade 3 or 4 mucositis. At this dose level 8 patients (53%) became tumour-free, 4 patients (26%) had marker normalization with irresectable residual disease and 2 patients were treatment failures. Though acute toxicity was severe at this dose level, there was no unexpected or unmanageable organ toxicity and thus patients are now entered at dose level 3, which consists of cisplatin 30 mg/m2, etoposide 200 mg/m2 and ifosfamide 1.6 g/m2 for 5 days and GMCSF 10 μg kg−1 day−1 on days 6–15 s.c.
Similar content being viewed by others
References
Antman KH, Griffin JD, Elias A, Socinski MA, Ryan L, Cannistra SA, Oette D, Whitley M, Frei E, Schnipper LE (1988) Effect of recombinant human granulocyte-macrophage colony-stimulating factor on chemotherapy induced myelosuppression. N Engl J Med 319:593
Birch R, Williams S, Cone A, et al. (1986) Prognostic factors for favourable outcome in disseminated germ cell tumors. J Clin Oncol 4:400
Bosl GJ, Geller NL, Vogelzang NJ, et al. (1987) Alternating cycles of etoposide plus cisplatin and VAB-6 in the treatment of poor risk patients with germ cell tumors. J Clin Oncol 5:436
Daugaard G, Roerth M (1986) High dose cisplatin and VP 16 with bleomycin in the management of advanced metastatic germ cell tumors. Eur J Cancer Clin Oncol 22:477
Einhorn L, Williams S, Loehrer PJ, et al. (1990) Phase III study of cisplatin dose intensity in advanced germ cell tumors: a Southeastern and Southwest Oncology Group research. Proc Am Soc Clin Oncol 9:510
Elias AD, Eder JP, Shea T, Begg CB, Frei E, Antman KH (1990) High dose ifosfamide with mesna uroprotection. J Clin Oncol 8:170
Horwich A, Brada M, Nicholls J, Jay G, Hendry WF, Dearnaly D, Peckham MJ (1989) Intensive induction chemotherapy for poor risk non-seminomatous germ cell tumors. Eur J Cancer Clin Oncol 25:177
Loehrer PJ, Einhorn LH, Williams SD (1986) VP-16 plus ifosfamide plus cisplatin as salvage therapy in refractory germ cell cancer. J Clin Oncol 4:528
Logothetis CJ, Samuels ML, Selig D, Swanson D, Johnson DE, Eschenbach AC von (1985) Improved survival with cyclic chemotherapy for nonseminomatous germ cell tumors of the testis. J Clin Oncol 3:326
Morstyn G, Burgess AW (1988) Hemopoietic growth factors: a review. Cancer Res 48:5624
Newlands ES, Begent RHJ, Rustin GJS, Parker D, Bagshave KD (1983) Further advances in the management of malignant teratomas of the testis and other sites. Lancet:948
Ozols R, Ihde DC, Linehan M, et al. (1988) A randomized trial of standard chemotherapy versus a high dose chemotherapy regimen in the treatment of poor prognosis germ cell tumors. J Clin Oncol 6:1031
Schmoll H-J, Arnoold H, Mayr A, et al. (1984) Platinum-ultra high dose/etoposide/bleomycin: an effective regimen for testicular cancer with poor prognosis. Proc Am Soc Clin Oncol 3:C639
Stoter G, Sleyfer DT, ten Bokkel Huinnink WW, et al. (1986) High dose vs low dose vinblastine in cisplatin-vinblastinebleomycin combination chemotherapy of nonseminomatous testicular cancer. J Clin Oncol 4:1199
Williams SD, Birch R, Einhorn LH (1987) Treatment of disseminated germ cell tumors with cisplatin, bleomycin and either vinblastine or etoposide. N Engl J Med 316:1435
Wolff SN, Johnson DH, Hainsworth JD, Greco A (1984) High dose VP-16-213 monotherapy for refractory germinal malignancies: a phase II study. J Clin Oncol 2:271
Author information
Authors and Affiliations
Additional information
Supported in part by Essex Pharma, München, FRG, and ASTA Medica, Frankfurt, FRG
For the Leitgruppe Hodentumoren; members: M. Bamberg, Tübingen; K. Behrendt, Essen; J. Hartlapp, Bonn; N. Jaeger, Bonn; N. Niederle, Essen; H.-J. Schmoll, Hannover; M. Wannenmacher, Freiburg; L. Weißbach, Berlin
Rights and permissions
About this article
Cite this article
Harstrick, A., Schmoll, H.J., Bokemeyer, C. et al. Cisplatin/etoposide/ifosfamide stepwise dose escalation with concomitant granulocyte/macrophage-colony-stimulating factor for patients with far-advanced testicular carcinoma. J Cancer Res Clin Oncol 117 (Suppl 4), S198–S202 (1991). https://doi.org/10.1007/BF01613227
Issue Date:
DOI: https://doi.org/10.1007/BF01613227