Summary
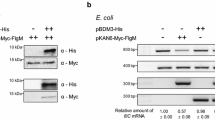
The nucleotide sequence of Salmonella abortus-equi fljA, which together with the phase 2 flagellin gene constitutes the fljBA operon and encodes the repressor for the phase 1 flagellin gene fliC, was determined. The repressor was predicted to be a basic protein consisting of 179 amino acid residues (Mr = 20419 Da) encoded by ORFII. This was confirmed by the fact that host fliC is repressed by plasmid-encoded ORFII, which indeed expresses a 20 kDa product as determined by urea SDS-polyacrylamide gel electrophoresis. An amino acid sequence capable of forming a helix-turn-helix type of structure was predicted in the C-terminal region of FljA. A rho-independent intercistronic terminator was detected between fljB and ftjA. Chloramphenicol acetyltransferase (CAT) assays of fusions indicated that the terminator is capable of reducing expression of fljA to the level of a few percent, relative to fljB in broth cultures and to 1 % in M9 glycerol cultures.
Similar content being viewed by others
References
An G, Bendiak D, Mamelak LA, Friesen JD (1981) Organization and nucleotide sequence of a new ribosomal operon in Escherichia coli containing the genes for ribosomal protein S2 and elongation factor Ts. Nucleic Acids Res 9:4163–4172
Barry G, Squires C, Squires CL (1980) Attenuation and processing of RNA from the rp1JL-rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci USA 77:3331–3335
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW, Cross JH, Falkow S (1977) Construction and characterization of new cloning vehicles II. A multi-purpose cloning system. Gene 2:95–113
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brennan RG, Matthews BW (1989) The Helix-Turn-Helix DNA binding motif. J Biol Chem 264:1903–1906
Burton ZF, Gross CA, Watanabe KK, Burgess RR (1983) The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell 32:335–349
Close TJ, Rodriguez RL (1982) Construction and characterization of the chloramphenicol-resistance gene cartridge: A new approach to the transcriptional mapping of extrachromosomal elements. Gene 20:305–316
Csonka LN, Clark AJ (1980) Construction of an Hfr strain useful for transferring recA mutations between Escherichia coli strains. J Bacteriol 143:529–530
Davis RW, Botstein D, Roth JR (1980) Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Defeyter RC, Davidson BE, Pittard J (1986) Nucleotide sequence of the transcription unit containing the aroL and aroM genes from Escherichia coli K-12. J Bacteriol 165:233–239
Dodd IA, Egan JB (1987) Systematic method for the detection of potential λ Cro-like DNA-binding regions in proteins. J Mol Biol 194:557–564
Enomoto M, Stocker BAD (1975) Integration, at hag or elsewhere, of H2 (phase-2 flagellin) genes transduced from Salmonella to Escherichia coli. Genetics 81:595–616
Enomoto M, Oosawa K, Momota H (1983) Mapping of the pin locus coding for a site-specific recombinase that causes flagellar-phase variation in Escherichia coli K-12. J Bacteriol 163:663–668
Enomoto M, Sakai A, Tominaga A (1985) Expression of an Escherichia coli flagellin gene, hag48, in the presence of a Salmonella III-repressor. Mol Gen Genet 201:133–135
Felmlee T, Pellett S, Welch RA (1985) Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol 163:94–105
Fujita H, Yamaguchi S, Iino T (1973) Studies of O-H variants in Salmonella in relation to phase variation. J Gen Microbiol 76:127–134
Gilbert W, Müller-Hill B (1970) The lactose repressor. In: Beckwith JR, Zipser D (eds) The lactose operon. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, p 93
Goldberger RF, Deeley RG, Mullinix KP (1976) Regulation of gene expression in prokaryotic organisms. Adv Genet 18:1–67
Hanafusa T, Sakai A, Tominaga A, Enomoto M (1989) Isolation and characterization of Escherichia coli hag operator mutants whose hag48 expression has become repressible by a Salmonella HI repressor. Mol Gen Genet 216:44–50
Harrison SC, Aggarwal AK (1990) DNA recognition by proteins with the Helix-Turn-Helix motif. Annu Rev Biochem 59:933–969
Hellinga HW, Evans PR (1985) Nucleotide sequence and high-level expression of the major Escherichia coli phosphofructokinase. Eur J Biochem 149:363–373
Iino T (1974) Assembly of Salmonella flagellin in vitro and in vivo. J Supramol Struct 2:372–384
Iino T (1977) Genetics of structure and function of bacterial flagella. Annu Rev Genet 11:166–182
Ishii S, Kuroki K, Imamoto F (1984) tRNA METf2 gene in the leader region of the nusA operon in Escherichia coli. Proc Natl Acad Sci USA 81:409–413
Iwakura Y, Ito K, Ishihama A (1974) Biosynthesis of RNA polymerase in Escherichia coli I. Mol Gen Genet 133:1–23
Kolter R, Yanofsky C (1982) Attenuation in amino acid biosynthetic operons. Annu Rev Genet 16:113–134
Komeda Y, Suzuki H, Ishidsu J, Iino T (1975) The role of CAMP in flagellation of Salmonella typhimurium. Mol Gen Genet 142:289–298
Lupski JR, Smiley BL, Godson GN (1983) Regulation of the rpsU-dnaG-rpoD macromolecular synthesis operon and the initiation of DNA replication in Escherichia coli K-12. Mol Gen Genet 189:48–57
Maloy SR, Nunn WD (1981) Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol 145:1110–1112
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: A laboratory manual Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Messing J (1983) New M13 vectors for cloning. Methods Enzymol 101:20–78
Pabo CO, Sauer RT (1984) Protein-DNA recognition. Annu Rev Biochem 53:293–321
Platt T (1986) Transcription termination and the regulation of gene expression. Annu Rev Biochem 55:339–372
Post LE, Strycharz GD, Nomura M, Lewis H, Dennis PP (1979) Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit β in Escherichia coli. Proc Natl Acad Sci USA 76:1697–1701
Ralling G, Linn T (1984) Relative activities of the transcriptional regulatory sites in the rplKAJLrpoBC gene cluster of Escherichia coli. J Bacteriol 158:279–285
Rodriguez RL, Tait RC (1983) Recombinant DNA techniques: An introduction. Addison-Wesley Publishing Company, London
Sacerdot C, Dessen P, Hershey JWB, Plumbridge JA, Grunberg-Manago M (1984) Sequence of the initiation factor IF2 gene: Unusual protein features and homologies with elongation factors. Proc Natl Acad Sci USA 81:7787–7791
Sanger FS, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schleif RF, Wensink PC (1981) Practical methods in molecular biology. Springer-Verlag, New York
Schnetz K, Toloczyki C, Rak B (1987) β-glucoside (bgl) operon of Escherichia coli K-12: Nucleotide sequence, genetic organization, and possible evolutionary relationship to regulatory components of two Bacillus subtilis genes. J Bacteriol 169:2579–2590
Shine J, Dalgarno L (1974) The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA 71:1342–1346
Silhavy TJ, Berman ML, Enquist LW (1984) Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Silverman M, Simon M (1974) Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J Bacteriol 120:1196–1203
Silverman M, Zieg J, Simon M (1979) Flagellar-phase variation: isolation of the rhl gene. J Bacteriol 137:517–523
Smiley BL, Lupski JR, Svec PS, McMacken R, Godson GN (1982) Sequences of the Escherichia coli dnaG primase gene and regulation of its expression. Proc Natl Acad Sci USA 79:4550–4554
Soberon X, Covarrubias L, Bolivar F (1980) Construction and characterization of new cloning vehicles IV. Deletion derivatives of pBR322 and pBR325. Gene 9:287–305
Stanley KK, Luzio JP (1984) Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J 3:1429–1434
Szekely E, Simon M (1983) DNA sequence adjacent to flagellar genes and evolution of flagellar-phase variation. J Bacteriol 155:74–81
Tiedeman AA, Smith JM (1985) Nucleotide sequence of the guaB locus encoding IMP dehydrogenase of Escherichia coli K12. Nucleic Acids Res 13:1303–1316
Tominaga A, Ikemizu S, Enomoto M (1991) Site-specific recombinase genes in three Shigella subgroups and nucleotide sequences of a pinB gene and an invertible B segment from Shigella boydii. J Bacteriol 173:4079–4087
Trachtenberg S, DeRosier DJ (1987) Three-dimensional structure of the frozen-hydrated flagellar filament. The left-handed filament of Salmonella typhimurium. J Mol Biol 195:581–601
Vieira J, Messing J (1987) Production of single-stranded plasmid DNA. Methods Enzymol 153:3–11
Yokota T, Gots JS (1970) Requirement of adenosine 3′, 5′-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol 103:513–516
Zieg J, Silverman M, Hilmen M, Simon M (1977) Recombination switch for gene expression. Science 196:170–172
Author information
Authors and Affiliations
Additional information
Communicated by K. Isono
Rights and permissions
About this article
Cite this article
Hanafusa, T., Saito, K., Tominaga, A. et al. Nucleotide sequence and regulated expression of the Salmonella fljA gene encoding a repressor of the phase 1 flagellin gene. Molec. Gen. Genet. 236, 260–266 (1993). https://doi.org/10.1007/BF00277121
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00277121