Abstract
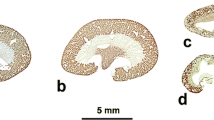
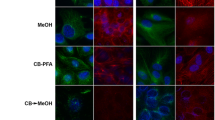
The spatial organization of the endoplasmic reticulum has been studied in two renal cell lines, MDCK and LLC-PK1, which originate from the distal and proximal portions of the mammalian nephron, respectively, and which form a polarized epithelium when they reach confluence in tissue culture. The two renal cell lines, grown to confluence on either solid or permeable supports, were investigated by fluorescence microscopy, confocal microscopy, and transmission electron microscopy. Fluorescence labeling of the endoplasmic reticulum was achieved using the cationic fluorescent dye DIOC6 (3). In order to differentiate fluorescent labeling of the endoplasmic reticulum from that of the mitochondria, cells were also labeled with rhodamine 123. For electron microscopy, the spatial organization of the endoplasmic reticulum was examined in thick sections using the long-duration osmium impregnation technique or the ferrocyanide/osmium technique. In both cell lines, the endoplasmic reticulum formed an abundant tubular network of canaliculi that frequently abutted the basolateral domain of the plasma membrane and occasionally the apical membrane. Elements of the endoplasmic reticulum were also found in close proximity to mitochondria that, as in the nephron, formed branched structures. Canaliculi appeared circular or flattened and had an inner diameter of 10–70 nm for MDCK cells and 20–90 nm for LLC-PK1 cells. Such a three-dimensional organization might facilitate the translocation of defined lipid species between the endoplasmic reticulum and the plasma membrane, and between the endoplasmic reticulum and mitochondria.
Similar content being viewed by others
References
Allen NS, Brown DT (1988) Dynamics of the endoplasmic reticulum in living onion epidermal cells in relation to microtubules, microfilaments, and intracellular particle movement. Cell Motil Cytoskelton 10:153–163
Bacallao R, Antony C, Dotti C, Karsenti E, Stelzer EHK, Simons K (1989) The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J Cell Biol. 109:2817–2832
Bergeron M, Thiéry G (1981) Three-dimensional characteristics of the endoplasmic reticulum of rat renal tubule cells, an electron microscopy study in thick-sections. Biol Cell 42:43–48
Bergeron M, Guérette D, Forget J, Thiéry G (1978) Three-dimensional characteristics of the endoplasmic reticulum of the nephron: a transcellular route. Kidney Int 13:102A
Bergeron M, Guérette D, Forget J, Thiéry G (1980) Three-dimensional characteristics of the mitochondria of the rat nephron. Kidney Int 17:175–185
Bergeron M, Gaffiero P, Thiéry G (1987) Segmental variations in the organization of the endoplasmic reticulum of the rat nephron. Cell Tissue Res 247:215–225
Bergeron M, Gaffiero, P Berthelet F, Thiéry G (1988) Interrelationship between organelles in kidney cells of adult and developing rat. Pediatric Nephrol 2:100–107
Berthelet F, Beaudry-Lonergan M, Linares H, Whittembury G, Bergeron M (1987) Polymorphic organization of the endoplasmic reticulum of the Malpighian tubule. Evidence for a transcellular route. Cellule 74:281–290
Cereijido M, Robbins ES, Dolan WJ, Rotunno CA, Sabatini DD (1977) Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol 77:853–880
Cereijido M, Contreras RG, Gonzales-Mariscal L (1989) Development and alteration of polarity. Ann Rev Physiol 51:785–795
De Bruyn WC (1968) A modified OsO4-double fixation procedure which selectively contrasts glycogen. 4iéme Congrès Européen Microscopie Électronique, Rome 1–7 September 1968; vol II, pp 65–66
Eilers U, Klumperman J, Hauri HP (1989) Nocodazole, a microtubule-active drug, interferes with apical protein delivery in cultured intestinal epithelial cells (Caco-2). J Cell Biol 108:13–22
Ericsson JLE, Trump BF (1964) Electron microscopic studies of the epithelium of the proximal tubule of the rat kidney. Lab Invest 11:1427–1456
Flores V, Lane NJ (1990) Evidence for a transcellular cisternal route across the caecal epithelium of an insect. Cell Tissue Res 261:347–354
Friedlander G, Shahedi M, Le Grimellec C, Amiel C (1988) Increase in membrane fluidity and opening of tight junctions have similar effects on sodium-coupled uptakes in renal epithelial cells. J Biol Chem 263:11183–11188
Giocondi MC, Le Grimellec C (1991) Water potassium in Madin-Darby canine kidney cells is modulated by membrane fluidity. Biochim Biophys Acta 1064:315–320
Goldstein S, Korczack LB (1981) Status of mitochondria in living human fibroblasts during growth and senescence in vitro: use of the laser dye rhodamine 123. J Cell Biol 91:392–398
Handler JS, Perkins FM, Johnson JP (1980) Studies of renal cell function using cell culture techniques. Am J Physiol 238:F1-F9
Hopkins CR, Gibson A, Shipman M, Miller K (1990) Movements of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature 346:335–339
Johnson LV, Walsh ML, Bockus BJ, Chen LB (1981) Monitoring of relative mitochondria membrane potential in living cells by fluorescence microscopy. J Cell Biol 88:526–535
Kaplan MR, Simoni RD (1985) Intracellular transport of phosphatidylcholine to the plasma membrane. J Cell Biol 101:441–445
Knebel W, Quader H, Schnepf E (1990) Mobile and immobile endoplasmic reticulum in onion bulb epidermis cells: short and long term observations with a confocal laser scanning microscope. Eur J Cell Biol 52:175–206
Kuismanen E, Saraste J (1989) Low temperature-induced transport blocks as tools to manipulate membrane traffic. Methods Cell Biol 32:257–274
Lee C, Chen LB (1988) Dynamic behavior of endoplasmic reticulum in living cells. Cell 54:37–46
Louvard D, Reggio H, Warren G (1982) Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol 92:92–107
Møllgård K, Rostgaard J (1978) Morphological aspects of some sodium transporting epithelia suggesting a transcellular pathway via elements of endoplasmic reticulum. J Membrane Biol 40:71–89
Munro S, Pelham HRB (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48:899–907
Pagano RE, Longmuir KJ, Martin OC, Struck DK (1981) Metabolism and intracellular localization of fluorescently labeled intermediate in lipid biosynthesis within cultured fibroblasts. J Cell Biol 91:872–877
Palade GE, Porter KR (1954) Studies on the endoplasmic reticulum. I. Its identification in cells in situ. J Exp Med 100:641–656
Porter KR (1953) Observations on a submicroscopic basophilic component of cytoplasm. J Exp Med 97:727–749
Preuss D, Mulholland J, Kaiser CA, Orlean P, Albright C, Rose MD, Robbins PW, Botstein D (1991) Structure of the yeast endoplasmic reticulum: localization of ER proteins using immunofluorescence and immunoelectron microscopy. Yeast 7:891–911
Qvortrup K, Rostgaard J (1990) Three-dimensional organization of a transcellular tubulocisternal endoplasmic reticulum in epithelial cells of Reissner's membrane in the guinea-pig. Cell Tissue Res 261:287–299
Rabito CA (1986) Occluding junctions in a renal cell line (LLC-PK1) with characteristics of proximal tubular cells. Am J Physiol 250:F734-F743
Rodriguez-Boulan E, Nelson WJ (1989) Morphogenesis of the polarized epithelial cell phenotype. Science 245:718–725
Sleight RG, Pagano RE (1983) Rapid appearance of newly synthesized phosphatidylethanolamine at the plasma membrane. J Biol Chem 258:9050–9058
Steele RE, Preston AS, Johnson JP, Handler JS (1986) Porous-bottom dishes for culture of polarized cells. Am J Physiol 251:C136-C139
Terasaki M (1990) Recent progress on structural interactions of the endoplasmic reticulum. Cell Motil Cytoskelton 15:71–75
Terasaki M, Sardet C (1991) Demonstration of calcium uptake and release by sea urchin egg cortical endoplasmic reticulum. J Cell Biol 115:1031–1037
Terasaki M, Song J, Wong JR, Weiss MJ, Chen LB (1984) Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell 38:101–108
Thiéry G (1979) Colorations signalétiques électives sur coupes épaisses du réticulum endoplasmique, de la chromatine et des surfaces cellulaires libres des cellules animales. Biol Cell 35:159–164
ThiéryG, Bergeron M (1976) Morphologie spatiale des mitochondries des tubes proximaux et distaux du néphron. Rev Can Biol 35:211–216
Thiéry G, Gaffiero P, Bergeron M (1983) Three-dimensional characteristics of the endoplasmic reticulum in the columnar cells of the rat small intestine. An electron microscopy study in thick sections. Am J Anat 167:479–493
Tooze J, Hollinshead M (1991) Tubular early endosomal networks in AtT20 and other cells. J Cell Biol 115:635–653
Vance JE (1991) Newly made phosphatidylserine and phosphatidylethanolamine are preferentially translocated between rat liver mitochondria and endoplasmic reticulum. J Biol Chem 266:89–97
Voelker DR (1991) Organelle biogenesis and intracellular lipid transport in eukaryotes. Microbiol Rev 55:543–560
Wijnaendts van Resandt RW, Marsman HJB, Kaplan R, Davoust J, Stelzer EHV, Stricker R (1985) Optical fluorescence microscopy in three dimensions: microtomoscopy. J Microscopy 138:29–34
Wijnaendts van Resandt RW, Ihrig C, Knebel W, Quader H (1989) 3D confocal microscopy of cytoskeleton structures. Eur J Cell Biol 48:39–42
Yaffe MP, Kennedy EP (1983) Intracellular phospholipid movement and the role of phospholipid transfer proteins in animal cells. Biochemistry 22:1497–1507
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bergeron, M., Thiéry, G., Lenoir, F. et al. Organization of the endoplasmic reticulum in renal cell lines MDCK and LLC-PK1. Cell Tissue Res 277, 297–307 (1994). https://doi.org/10.1007/BF00327777
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00327777