Summary
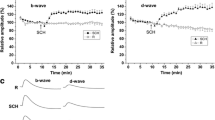
The cardiac glycoside ouabain was injected into the eye-bulb of the teleost fish, Carassius carassius. Three doses of ouabain were used: 10-4 M, 10-5 M, 10-6 M. The final concentrations in the vitreous body of the eye were approximately 3 · 10-5 M, 3 · 10-6 M and 3 · 10-7 M, respectively. After 8 hrs, 1,2,4,6 and 8 days the ultrastructural alterations of retinal ganglion cells, the optic axons near the bulb and the terminal segments in the optic tectum were studied. The high doses of ouabain induced an early necrobiosis of the cell bodies in the retina followed by degeneration in the nerve. This is characterized as a protracted form of Wallerian degeneration. The significance of the inhibition of Na+-K+-activated ATPase at the perikaryal level for both the integrity of axonal morphology and the axonal flow is discussed.
Similar content being viewed by others
References
Akera, T., Hook, J.B., Tobin, T., Brody, T.M.: Cardiac glycoside sensitivity of (Na+, K+)-activated ATPase in new-born rats. Res. Comm. Chem. Path. Pharmacol. 4, 699–706 (1972)
Aldskogius, H.: Indirect and direct Walierian degeneration in the intramedullary root fibres of the hypoglossal nerve. An electron microscopical study in the kitten. Advanc. Anat. Embryol. Cell Biol. 50, 1–78 (1974)
Anderson, K.-E., Edström, A.: Effects of nerve blocking agents on fast axonal transport of proteins in frog sciatic nerves in vitro. Brain Res. 50, 125–134 (1973)
Barondes, S.H.: Neuronal proteins, synthesis, transport and neuronal regulation. In: Proteins of the nervous system (eds. D.J. Schneider, R.H. Angeletti, R.A. Bradshaw, A. Grasso and B.W. Moore), p. 215–226. New York: Raven Press, 1973
Borle, A.B.: Calcium and phosphate metabolism. Ann. Rev. Physiol. 36, 361–390, (1974)
Brimijoin, S., Capek, P., Dyck, P.J.: Axonal transport of dopamine-β-hydroxylase by human sural nerves in vitro. Science 180, 4092, 1295–1297 (1973)
Cook, R.D., Ghetti, B., Wísniewski, H.M.: The pattern of Wallerian degeneration in the optic nerve of newborn kittens: an ultrastructural study. Brain Res. 75, 261–276 (1974)
Dunham, E.T., Glynn, J.M.: Adenosinetriphosphatase activity and the active movements of alkali metal ions. J. Physiol. (Lond.) 156, 274–293 (1961)
Edström, A.: Ionic requirements for rapid axonal transport in vitro in frog sciatic nerves. Acta physiol. scand. 93, 104–112 (1975)
Edström, A., Hansson, H.-A., Norström, A.: Inhibition of axonal transport in vitro in frog sciatic nerves by chlorpromazine and lipocaine. A biochemical and ultrastructural study. Z. Zellforsch. 143, 53–69 (1973)
Erdmann, E., Schoner, W.: Ouabain-receptor interactions in (Na++ K+)-ATPase preparations. II. Effect of cations and nucleotides on rate constants and dissociation constants. Biochim. biophys. Acta (Amst.) 330, 302–315 (1973)
Feit, H., Barondes, S.H.: Colchicine-binding activity in paniculate fractions of mouse brain. J. Neurochem. 17, 1355–1364 (1970)
Feit, H., Dutton, G.R., Barondes, S.H., Shelanski, M.L.: Microtubule protein: identification in and transport to nerve endings. J. Cell Biol. 51, 138–147 (1971)
Fink, B.R., Byers, M.R., Middaugh, M.E.: Dynamics of colchicine effects on rapid axonal transport and axonal morphology. Brain Res. 56, 299–312 (1973)
Frizell, M., McLean, W.G., Sjöstrand, J.: Slow axonal transport of proteins; blockade by interruption of contact between cell body and axon. Brain Res. 86, 67–73 (1975)
Garcia, A.G., Kirpekar, S.M., Prat, J.C., Wakade, A.R.: Metabolic and ionic requirements for the axoplasmic transport of dopamine β-hydroxylase. J. Physiol. (Lond.) 241, 809–821 (1974)
Glynn, J.M.: The action of cardiac glycosides on ion movements. Pharmacol. Rev. 16, 381–407 (1964)
Grafstein, B.: Transport of protein by goldfish optic nerve fibers. Science 157, 196–198 (1967)
Grafstein, B., McEwen, B.S., Shelanski, M.L.: Axonal transport of neurotubule protein. Nature (Lond.) 227, 289 (1970)
Hall, S.M.: The effects of injection of potassium cyanide into the sciatic nerve of the adult mouse: in vivo and electron microscopical studies. J. Neurocytol. 1, 233–254 (1972)
Hirlinger, W.K.: Nucleo-distaler Beginn der Axonveränderungen bei sekundärer Waller'scher Faserdegeneration. Quantitative elektronenmikroskopische Beobachtungen am Nervus und Tractus opticus der Ratte. Dissertation Tübingen 1973
Ingoglia, N.A., Grafstein, B., McEwen, B.S., McQuarrie, L.G.: Axonal transport of radioactivity in the goldfish optic system following intraocular injection of labelled RNA precursors. J. Neurochem. 20, 1605–1616 (1973)
Joseph, B.S.: Somatofugal events in Wallerian degeneration: a conceptual overview. Brain Res. 59, 1–18 (1973)
Karlsson, J.-O., Sjöstrand, J.: Axonal transport of proteins in retinal ganglion cells. Characterization of the transport to the superior colliculus. Brain Res. 47, 185–194 (1972)
Kreutzberg, G.: Neuronal dynamics and axonal flow. IV. Blockage of intraaxonal enzyme transport by colchicine. Proc. nat. Acad. Sci. (Wash.) 62, 722–728 (1969)
Kruger, L., Maxwell, D.S.: Wallerian degeneration in the optic nerve of a reptile: an electron microscopic study. Amer. J. Anat. 125, 247–270 (1969)
Lassek, A.M., Hard, W.L.: The pyramidal tract. The relation of axonal diameters to the rate of degeneration as revealed by the acid phosphatase method in the monkey. J. Neuropath. exp. Neurol. 5, 374–379 (1946)
Laufer, M., Vanegas, H.: The optic tectum of a perciform teleost. II. Fine structure. J. comp. Neurol. 154, 61–96 (1974a)
Laufer, M., Vanegas, H.: The optic tectum of a perciform teleost. III. Electron microscopy of degenerating retino-tectal afferents. J. comp. Neurol. 154, 97–116 (1974b)
Lee, J.C.-Y.: Electron microscopy of Wallerian degeneration. J. comp. Neurol. 120, 65–71 (1963)
Lee, Y.C., Samson, F.E., Houston, L.L., Himes, R.H.: The in vitro polymerization of tubulin from beef brain. J. Neurobiol. 5, 317–330 (1974)
McEwen, B.S., Grafstein, B.: Fast and slow components in axonal transport of protein. J. Cell Biol. 38, 494–508 (1968)
Neale, J.H., Neale, E.A., Agranoff, B.W.: Radioautography of the optic tectum of the goldfish after intraocular injection of 3H-proline. Science 176, 407–409 (1972)
Ochs, S., Hollingsworth, D.: Dependence of fast axoplasmic transport in nerve on oxidative metabolism. J. Neurochem. 18, 107–114 (1971)
Rahmann, H.: Entstehungsorte und Verbleib von Syntheseprodukten im Zentralnervensystem von Vertebraten. Zool. Anz. Suppl. 33, (Verh. Zool. Ges. 1969) 430–460 (1969)
Rahmann, H.: Different modes of substances flow in the optic tract. Acta neuropath. Suppl. V, 162–170 (1971)
Reier, P.J., Webster, H de F.: Regeneration of Xenopus tadpole optic nerve fibres following transsection or crush. J. Neurocytol. 3, 591–618 (1974)
Richards, W., Kalil, R.: Dissociation of retinal fibers by degeneration rate. Brain Res. 72, 288–293 (1974)
Rosenberg, H.M.: Variant HeLa-cells selected for their resistance to ouabain. J. Cell. Physiol. 85, 135–142 (1975)
Sabri, M.I., Ochs, S.: Relation of ATP and creatine phosphate to fast axoplasmic transport in mammalian nerve. J. Neurochem. 19, 2821–2828 (1972)
Schlaepfer, W.W.: Experimental alterations of neurofilaments and neurotubules by calcium and other ions. Exp. Cell Res. 67, 73–80 (1971)
Schlaepfer, W.W.: Calcium-induced degeneration of axoplasm in isolated segments of rat peripheral nerve. Brain Res. 69, 203–215 (1974a)
Schlaepfer, W.W.: Effects of energy deprivation on Wallerian degeneration in isolated segments of rat peripheral nerve. Brain Res. 78, 71–81 (1974b)
Schlaepfer, W.W., Bunge, R.P.: Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J. Cell Biol. 59, Part 1, 456–470 (1973)
Schlote, W.: Nervus opticus und experimentelles Trauma. Monographien aus dem Gesamtgebiete der Neurologie und Psychiatrie. Bd. 131, Berlin-Heidelberg-New York: Springer 1970
Schonbach, J., Cuenod, M.: Axoplasmic migration of protein. A light microscopical autoradiographic study in the avian retinotectal pathway. Exp. Brain Res. 12, 275–282 (1971)
Towfighi, J., Gonatas, N.K.: Effect of intracerebral injection of ouabain in adult and developing rats: an ultrastructural and autoradiographic study. Lab. Invest. 28, 170–180 (1973)
Trump, B.F., Croker, B.P., Mergner, W.J.: The role of energy metabolism, ion, and water shifts in the pathogenesis of cell injury. In: Cell membranes. Biological and pathological aspects (ed. G.W. Richter, D.G. Scarpelli) p. 84–128. Baltimore: Williams and Wilkins Company 1971
Turner, J.E., Singer, M.: The ultrastructure of Wallerian degeneration in the severed optic nerve of the newt (Triturus viridescens). Anat. Rec. 181, 267–286 (1975)
Vanegas, H., Laufer, M., Amat, J.: The optic tectum of a perciform teleost. I. General configuration and cytoarchitecture. J. comp. Neurol. 154, 43–60 (1974)
Wawrzyniak, M.: Chemoarchitektonische Studien am Tectum opticum von Teleostiern unter normalen und experimentellen Bedingungen. Z. Zellforsch. 58, 234–264 (1962)
Weiss, P.: The concept of perpetual neuronal growth and proximodistal substance convection. 4th. International Neurochemical Symposium, p. 220–242. New York: Pergamon Press 1960
Weiss, P., Hiscoe, H.B.: Experiments on the mechanism of nerve growth. J. exp. Zool. 107, 315–396 (1948)
Willard, M., Cowan, W.M., Vagelos, P.R.: The polypeptide composition of intra-axonally transported proteins: evidence for four transport velocities. Proc. nat. Acad. Sci. (Wash.) 71, 3–2187 (1974)
Wolburg, H.: Intraaxonaler Transport von Ethidium-Bromid-sensitiven RNS- und niedermolekularen 3H-Uridin-Verbindungen im Tractus opticus von Teleosteern. Exp. Brain Res. 15, 348–363 (1972)
Author information
Authors and Affiliations
Additional information
This work was supported by the grant Wo 215/1 of the Deutsche Forschungsgemeinschaft. I am gratefully indebted to Prof. Dr. W. Schlote for helpful discussions and for reading the manuscript.
Rights and permissions
About this article
Cite this article
Wolburg, H. Time- and dose-dependent influence of ouabain on the ultrastructure of optic neurones. Cell Tissue Res. 164, 503–517 (1975). https://doi.org/10.1007/BF00219941
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00219941