Summary
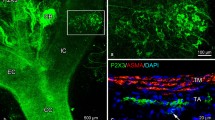
The proximal truncus arteriosus of the lizard Trachydosaurus rugosus was studied with light-, fluorescence and electron-microscopical techniques. Three vessels comprised the truncus: the pulmonary, left aortic, and caroticoaortic arteries. Right and left truncal nerves, each derived from the ipsilateral vagus nerve, innervated the truncus, particularly its proximal 3 mm.
Ultrastructurally, the nerves had a variety of appearances: some were clearly adrenergic, c-type or p-type. A number of profiles contained large numbers of mitochondria and were classified as sensory. Some profiles defied exact classification, having characteristics common to two different types of profile.
Within the outer medial layers, profiles up to 7 μm in diameter were found. These contained large numbers of mitochondria, myelin bodies and structures intermediate between the two. In addition, the profiles contained large amounts of glycogen and small numbers of vesicles. These nerve fibres were classified as baroreceptors, since they closely resemble carotid sinus and aortic arch baroreceptors in mammals.
Large numbers of chromaffin cells were found, particularly in the common wall of the pulmonary and left aortic arteries. Many of these cells emitted a long tapering process, which sometimes entered a nearby nerve bundle. Sensory, p-type and c-type profiles, but not adrenergic profiles, made extensive close contacts with chromaffin cells.
Similar content being viewed by others
References
Adams WE (1962) The carotid sinus-carotid body problem in the Chelonia (with a note on a foramen of Panizza in Dermochelys). Arch Int Pharmacodyn Ther 139:28–37
Akert K, Sandri C (1968) An electron microscope study of zinc iodide-osmium impregnation of neurons. I. Staining of synaptic vesicles at cholinergic junctions. Brain Res 7:286–295
Baumgarten HG, Holstein AF, Owman C (1970) Auerbach's plexus of mammals and man: Electron microscopic identification of three different types of neuronal processes in myenteric ganglia of the large intestine from rhesus monkeys, guinea-pigs and man. Z Zellforsch 106:376–397
Berger PJ, Evans BK, Smith DG (1980) Localisation of baroreceptors and gain of the baroreceptor heart rate reflex in the lizard Trachydosaurus rugosus. J Exp Biol 86:197–209
Blest AD (1976) A new method for the reduced silver impregnation of arthropod central nervous system. Proc R Soc Lond [cmBiol] 193:191–197
Böck P, Gorgas K (1976) Fine structure of baroreceptor terminals in the carotid sinus of guinea-pigs and mice. Cell Tissue Res 170:98–112
Chiba T (1972) Fine structure of the baroreceptor nerve terminals in the carotid sinus of the dog. J Electron Microsc (Tokyo) 21:139–148
Conn HJ, Darrow MA, Emmel VM (1960) Staining procedures. Williams & Wilkins, Baltimore, 2nd edn, p 66
Düring M von, Miller MR (1979) Sensory nerve endings of the skin and deeper structures. In: Gans C (ed) Biology of the reptilia 9 neurology A. Academic Press, London New York San Francisco, pp 407–441
Düring M von, Andres KH, Iravai J (1974) The fine structure of the pulmonary stretch receptor in the rat. Z Anat Entwicklungsgesch 143:215–222
Fedele M (1937) I nervi del tronco arterioso nei quadro della innervazioni cardiaca nei Rettili e il problema del “depressore” nei Vertebrata. Mem R Acc Naz Lincei Ser 6, 6:387–520
Fidone S, Stensaas L, Zapata P (1975) Sensory nerve endings containing “synaptic” vesicles: An electron microscopic study. J Neurobiol 6:423–427
Fillenz M, Woods RI (1970) Sensory innervation of the airways. In: Porter R (ed) Breathing: Hering Breuer Symposium. Ciba Foundation, Churchill London, pp 101–109
Furness JB, Moore J (1970) The adrenergic innervation of the cardio-vascular system of the lizard Trachysaurus rugosus. Z Zellforsch 108:150–176
Furness JB, Sobels G (1976) The ultrastructure of paraganglia associated with the inferior mesenteric ganglia in the guinea-pig. Cell Tissue Res 171:123–139
Gibbins IL (1982) Lack of correlation between ultrastructural and pharmacological types of nonadrenergic autonomic nerves. Cell Tissue Res 221:551–581
Haller CJ, Rogers DC (1978) The innervation and fine structure of paraneuronic cells in an amphibian pulmonary artery. Cell Tissue Res 195:411–423
Hamberger B, Malmfors T, Sachs C (1965) Standardisation of paraformaldehyde and of certain procedures for the histochemical demonstration of catecholamines. J Histochem Cytochem 13:147
Hansen JJ (1979) An ultrastructural stereological analysis of the aortic body chief cells of adult rabbits. Cell Tissue Res 196:511–518
Hartschuh W, Grube D (1979) The Merkel cell — a member of the APUD system? Arch Dermatol Res 265:115–122
Humason GL (1972) Animal tissue techniques, 3rd edn. Freeman WH & Co, San Francisco, pp 14–18
Iggo A (1976) Is the physiology of cutaneous receptors determined by morphology? Prog Brain Res 43:15–31
Jonsson G (1971) Quantitation of fluorescence of biogenic monoamines demonstrated with the formaldehyde fluorescence method. Prog Histochem Cytochem 2:299–334
King AS, McLelland J, Cook RD, King DZ, Walsh C (1974) The ultrastructure of afferent nerve endings in the avian lung. Respir Physiol 22:21–40
Kirby S, Burnstock G (1969) Comparative pharmacological studies of isolated spiral strips of large arteries from lower vertebrates. Comp Biochem Physiol 28:307–319
Knoche H, Addicks K (1976) Electron microscopic studies of the pressoreceptor fields of the carotid sinus of the dog. Cell Tissue Res 173:77–94
Kondo H (1976) Innervation of the carotid body of the adult rat. A serial ultrathin section analysis. Cell Tissue Res 173:1–15
Kondo H (1980) Is the SIF cell truly an interneuron in the superior cervical ganglion? Adv Biochem Psychopharmacol 25:103–110
Krauhs JM (1979) Structure of rat aortic baroreceptors and their relationships to connective tissue. J Neurocytol 8:401–414
Lahiri S, Nishino T, Mokashi A, Mulligan E (1980) Interaction of dopamine and haloperidol with O2 and CO2 chemoreception in carotid body. J Appl Physiol 49:45–51
Landeman L, Halata Z (1980) Merkel cells and nerve endings in the labial epidermis of a lizard. Cell Tissue Res 210:353–357
Mahony R (1973) Laboratory Techniques in Zoology, 2nd edn. Butterworth & Co, London, pp 267–268
Matthews MR (1980) Ultrastructural studies relevant to the possible function of small granulecontaining cells in the rat superior cervical ganglion. Adv Biochem Psychopharmacol 25:77–86
McDonald DM (1980) Regulation of chemoreceptor sensitivity in the carotid body: the role of presynaptic sensory nerves. Fed Proc 39:2627–2635
McDonald DM, Mitchell RA (1975) The innervation of glomus cells, ganglion cells and blood vessels in the rat carotid body: A quantitative ultrastructural analysis. J Neurocytol 4:177–230
Mills E, Smith PG, Slotkin TA, Breese G (1978) Role of carotid body catecholamines in chemoreceptor function. Neurosci 3:1137–1146
Nagatsu I, Kojima H, Inagaki S, Kondo Y, Nagatsu T (1979) Immunohistochemical localisation of catecholamine synthesising enzymes in bullfrog adrenals. Acta Histochem Cytochem 12:416–422
Nishi K, Stensaas LJ (1974) The ultrastructure and source of nerve endings in the carotid body. Cell Tissue Res 154:303–319
Ottaviani G, Tazzi A (1977) The lymphatic system. In: Gans C, Parsons TS (eds) Biology of the reptilia 6 morphology E. Academic Press, London New York San Francisco, pp 315–462
Rees PM (1967) Observations on the fine structure and distribution of presumptive baroreceptor nerves at carotid sinus. J Comp Neurol 131:517–548
Scalzi HA, Price HM (1971) The arrangement and sensory innervation of the intrafusal fibres in the feline muscle spindle. J Ultrastruct Res 36:375–390
Smith KR (1967) The structure and function of the Haarscheibe. J Comp Neurol 131:459–474
Smith PG, Mills E (1979) Physiological and ultrastructural observations on regenerated carotid sinus nerves after removal of the carotid bodies. Neurosci 4:2009–2020
Taxi J (1979) The chromaffin and chromaffin-like cells in the autonomic nervous system. Int Rev Cytol 57:283–343
Tranzer JP, Richards JG (1976) Ultrastructural cytochemistry of biogenic amines in nervous tissue: Methodologic improvements. J Histochem Cytochem 24:1178–1193
Trinci G (1912) Il sistema cromaffine cardiaco-cervicale nei Sauri. Arch Ital Anat Embryol 10:197–260
Uehara Y, Hama K (1965) Some observations on the fine structure of the frog muscle spindle. I. On the sensory terminals and motor endings of the muscle spindles. J Electron Microsc (Tokyo) 14:34–46
Verna A (1979) Ultrastructure of the carotid body in mammals. Int Rev Cytol 60:271–330
Verna A, Roumy M, Leitner L-M (1980) Role of the carotid body cells: long-term consequences of their cryodestruction. Neurosci Lett 16:281–285
White FN (1976) Circulation. In: Gans C, Dawson WR (eds) Biology of the reptilia 5 physiology A. Academic Press, London New York San Francisco, pp 275–334
Willis AG (1954) New methods for staining nerve fibres in pathological materials. J Pathol 68:277–283
Willis AG (1961) The argentophile reaction in elective histological analysis. Ann Histochim 6:527–536
Yates RD, Chen I-L (1980) An electron microscopic study of the baroreceptors in the internal carotid artery of the spontaneously hypertensive rat. Cell Tissue Res 205:473–483
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berger, P.J., Gibbins, I.L., Hards, D.K. et al. The distribution and ultrastructure of sensory elements in the baroreceptor region of the truncus arteriosus of the lizard Trachydosaums rugosus . Cell Tissue Res. 226, 389–406 (1982). https://doi.org/10.1007/BF00218368
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00218368