Summary
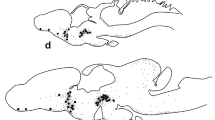
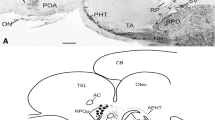
The peroxidase-antiperoxidase immunocytochemical procedure was used to study the distribution of ovine corticotropin-releasing factor (CRF) and arginine vasotocin (AVT) immunoreactivities sequentially in the same sections or in adjacent sections of the brain and pituitary of Catostomus commersoni. It was found that all CRF-immunoreactive (IR) neurons in the nucleus preopticus (NPO) also contained AVT immunoreactivity. Co-localization of both immunoreactivities was also observed in fibres forming the preoptic-pituitary tract and in the neurohypophyseal digitations, the IR-CRF and IR-AVT fibres projecting mainly to the neurointermediate lobe (NIL) of the pituitary. An additional population of exclusively IR-AVT neurons and fibres in the NPO, preoptic-pituitary tract and NIL was also observed. Exclusive CRF-immunostaining was found in neurons of the nucleus lateralis tuberis (NLT), in fibres distributed in some diencephalic nuclei and in the neurohypophyseal digitations in the region of the rostral pars distalis (RPD). These results suggest (i) that CRF- and AVT-like substances, present in NIL fibres (probably originating in the NPO), may have an integrated role in the release of the cell products from the pars intermedia, and (ii) that the control of corticotrops in the rostral pars distalis, innervated exclusively by IR-CRF fibres (probably originating in the NLT), does not require a simultaneous presence of CRF- and AVT-like substances.
Similar content being viewed by others
References
Buckingham JCV (1981) The influence of vasopressin on hypothalamic corticotropin releasing activity in rats with inherited diabetes insipidus. J Physiol 312:9–16
Bugnon C, Cardot J, Gouget A, Fellman D (1983) Mise en évidence d'un système neuronal peptidergique réactif à un immunosérum anti-CRF 41 chez les Téléostéens duleicules et marins. CR Acad Sci Paris 296 Ser III:711–716
Burford G, McLean L, Moore G, Lederis K (1977) Production and characterization of antisera to arginine vasotocin. Proc Can Fed Biol Soc [Abstr] 20:94
Fellman D, Bugnon C, Bresson JL, Gouget A, Cardot J, Clavequin MC, Hadjiyiasemis M (1984) The CRF neuron: Immunocytochemical study. Peptides [Suppl] 5:19–33
Fryer JN (1983) A light- and electron-microscopic study of goldfish corticotrophs following lesions of the nucleus praeopticus. Cell Tissue Res 234:31–38
Fryer JN, Lederis K (1985) Urotensin I and corticotropin secretion: Comparative actions in fishes and mammals. In: Kobayashi H, Bern HA, Urano A (eds) Neurosecretion and the Biology of Neuropeptides. Japan Science Society Press, Tokyo, Springer, Berlin Heidelberg New York, pp 464–470
Fryer JN, Maler L (1981) Hypophysiotropic neurons in the gold-fish hypothalamus demonstrated by retrograde transport of horseradish peroxidase. Cell Tissue Res 218:93–102
Fryer JN, Peter RE (1977a) Hypothalamic control of ACTH secretion in goldfish. I. Corticotropin-releasing factor activity in teleost brain tissue extracts. Gen Comp Endocrinol 33:196–201
Fryer JN, Peter RE (1977b) Hypothalamic control of ACTH secretion in goldfish. II. Hypothalamic lesioning studies. Gen Comp Endocrinol 33:202–214
Gillies GE, Linton EA, Lowry PJ (1982) Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature 299:355–357
Goosens N, Dierickx K, Vandesande F (1977) Immunocytochemical localization of vasotocin and isotocin in the preopticohypophyseal neurosecretory system of teleosts. Gen Comp Endocrinol 32:371–375
Kaul S, Vollrath L (1974a) The goldfish pituitary. I: Cytology. Cell Tissue Res 154:211–230
Kaul S, Vollrath L (1974b) The goldfish pituitary. II: Innervation. Cell Tissue Res 154:231–249
Kiss JZ, Mezey E, Skirboll L (1984) Corticotropin-releasing factor-immunoreactive neurons of the paraventricular nucleus become vasopressin positive after adrenalectomy. Proc Natl Acad Sci USA 81:1854–1858
Lamberts SWJ, Verleun T, Oosterom R, DeJong F, Hackeng WHL (1984) Corticotropin-releasing factor (ovine) and vasopressin exert a synergistic effect on adrenocorticotropin release in man. J Clin Endocrinol Metab 58:298
Larsson L-I (1981) A novel immunocytochemical model system for specificity and sensitivity screening of antisera against multiple antigens. J Histochem Cytochem 29:408–410
Lederis K, Fisher AW, Geonzon RM, Gill V, Ko D, Raghavan S (1980) Arginine vasotocin in fetal, newborn and adult mammals: evolution in progress. In: Ishii S, Hirano T, Wada M (eds) Hormones, Adaptation and Evolution. Japan Science Society Press, Tokyo/Springer, Berlin Heidelberg New York, pp 71–77
Lederis K, Fryer JN, Yulis CR (1985) The fish neuropeptide urotensin I: Its physiology and pharmacology. Peptides 6:353–361
Naito N, Takahashi A, Nakai Y, Kawauchi H (1984) Immunocytochemical identification of the proopiocortin-producing cells in the chum salmon pituitary with antisera to endorphin and NH2-terminal peptide of salmon proopiocortin. Gen Comp Endocrinol 56:185–192
Nozaki M, Gorbman A (1983) Immunocytochemical localization of somatostatin and vasotocin in the brain of the pacific hagfish, Eptatretus stouti. Cell Tissue Res 229:541–550
Olivereau M, Ollevier F, Vandesande F, Verdonck W (1984) Immunocytochemical identification of CRF-like and SRIF-like peptides in the brain and the pituitary of cyprinid fish. Cell Tissue Res 237:379–382
Ollevier F, Verdonck W (1984) Corticotropin-releasing-like factor in the pituitary of Salmo gairdneri. Gen Comp Endocrinol 53:433
Péczely P, Antoni FA (1984) Comparative localization of neurons containing ovine corticotropin releasing factor (CRF)-like and neurophysin-like immunoreactivity in the diencephalon of the pigeon (Columba livia domestica). J Comp Neurol 228:69–80
Peter RE, Fryer JN (1983) Endocrine functions of the hypothalamus of actinopterygians. In: Davis RE, Northcutt RG (eds) Fish Neurobiology. Volume 2. The University of Michigan Press, Ann Arbor, pp 165–201
Rodriguez K, Sumpter JP (1983) The distribution of some proopiomelanocortin-related peptides in the pituitary gland of the rainbow trout Salmo gairndneri. Gen Comp Endocrinol 51:454–459
Sakly M, Schmitt G, Koch B (1982) CRF enhances release of both alpha-MSH and ACTH from anterior and intermediate pituitary. Neuroendocrinol Lett 4:289–293
Sawchenko PE, Swanson LW, Vale WW (1984) Corticotropin-releasing factor: Co-expression within distinct subsets of oxytocin-vasopressin-, and neurotensin-immunoreactive neurons in the hypothalamus of the male rat. J Neurosci 4:1118–1129
Sofroniew MV, Weindl A, Shinko I, Wetzstein R (1979) The distribution of vasopressin-, oxytocin-, and neurophysin-producing neurons in the guinea pig brain. I. The classical hypothalamoneurohypophyseal system. Cell Tissue Res 197:367–384
Sternberger LA, Hardy PH, Cuculis JJ, Meyer HG (1970) The unlabeled antibody enzyme method of immunohistochemistry: Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antiperoxidase) and its use in identification of spirochetes. J Histochem Cytochem 18:315–333
Swaab DF, Pool CW (1975) Specificity of oxytocin and vasopressin immunofluorescence. J Endocrinol 66:263–272
Tramu G, Pillez A, Leonardelli J (1978) An efficient method of antibody elution for the successive or simultaneous localization of two antigens by immunocytochemistry. J Histochem Cytochem 26:322–324
Tramu G, Croix C, Pillez A (1983) Ability of the CRF immunoreactive neurons of the paraventricular nucleus to produce a vasopressin-like material. Neuroendocrinology 37:467–469
Van Den Dungen HM, Buijs RM, Pool CW, Terlou M (1982) The distribution of vasotocin and isotocin in the brain of the rainbow trout. J Comp Neurol 212:146–157
Yulis CR, Lederis K, Wong KL, Fisher AWF (1986) Localization of urotensin I- and corticotropin-releasing factor-like immunoreactivity in the central nervous system of Catostomus commersoni. Peptides 7:79–86
Zambrano D (1970) The nucleus lateralis tuberis system of the gobiid fish Gillichthys mirabilis. II. Innervation of the pituitary. Z Zellforsch 110:496–516
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yulis, C.R., Lederis, K. Co-localization of the immunoreactivities of corticotropin-releasing factor and arginine vasotocin in the brain and pituitary system of the teleost Catostomus commersoni . Cell Tissue Res. 247, 267–273 (1987). https://doi.org/10.1007/BF00218308
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00218308