Summary
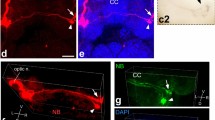
Retinopetal neurons were visualised in the telencephalon and diencephalon of an air-breathing teleost fish, Channa punctata, following administration of cobaltous lysine to the optic nerve. The labelled perikarya (n=45–50) were always located on the side contralateral to the optic nerve that had received the neuronal tracer. The rostral-most back-filled cell bodies were located in the nucleus olfactoretinalis at the junction between the olfactory bulb and the telencephalon. In the area ventralis telencephali, two groups of telencephaloretinopetal neurons were identified near the ventral margin of the telencephalon. The rostral hypothalamus exhibited retrogradely labelled cells in three discrete areas of the lateral preoptic area, which was bordered medially by the nucleus praeopticus periventricularis and nucleus praeopticus, and laterally by the lateral forebrain bundle. In addition to a dorsal and a ventral group, a third population of neurons was located ventral to the lateral forebrain bundle adjacent to the optic tract. The dorsal group of neurons exhibited extensive collaterals; a few extended laterally towards the lateral forebrain bundle, whereas others ran into the dorsocentral area of the area dorsalis telencephali. A few processes extended via the anterior commissure into the telencephalon ipsilateral to the optic nerve that had been exposed to cobaltous lysine. However, the ventral cell group did not possess collaterals. In the diencephalon, retinopetal cells were visualised in the nucleus opticus dorsolateralis located in the pretectal area; these were the largest retinopetal perikarya of the brain. The caudal-most nucleus that possessed labelled somata was the retinothalamic nucleus; it contained the largest number of retinopetal cells. The limited number of widely distributed neurons in the forebrain, some with extensive collaterals, might participate in functional integration of different brain areas involved in feeding, which in this species is influenced largely by taste, not solely by vision.
Similar content being viewed by others
References
Angaut P, Repérant J (1978) A light and electron-microscopic study of the nucleus isthmo-opticus in the pigeon. Arch Anat Microsc Morphol Exp 67:63–78
Bartheld CS von, Meyer DL (1986) Tracing of single fibres of the nervus terminalis in the goldfish brain. Cell Tissue Res 245:143–158
Bartheld CS von, Meyer DL (1988) Retinofugal and retinopetal projections in the teleost Channa micropeltes (Channiformes). Cell Tissue Res 251:651–663
Bartheld CS von, Rickman MJ, Meyer DL (1986) A light- and electron-microscopic study of mesencephalic neurons projecting to the ganglion of the nervus terminalis in the goldfish. Cell Tissue Res 246:63–70
Bhimachar BS (1935) A study of the correlation between the feeding habits and the structure of the hind brain in the South Indian cyprinoid fishes. Proc R Soc Lond (Biol) 117:258–272
Carter GS (1957) Air breathing. In: Brown ME (ed) The physiology of fishes, vol I, Metabolism. Academic Press, New York, pp 65–79
Crapon de Caprona MD, Fritzsch B (1983) The development of the retinopetal nucleus olfactoretinalis of two cichlid fish as revealed by horseradish peroxidase. Dev Brain Res 11:281–301
Crossland WJ (1979) Identification of tectal synaptic terminals in the avian isthmo-optic nucleus. In: Gronda AM, Maxwell JH (eds) Neural mechanisms and behavior in the pigeon. Plenum Press, New York, pp 267–285
Demski LS, Dulka JG (1984) Functional-anatomical studies on sperm release evoked by electrical stimulation of the olfactory tract in goldfish. Brain Res 291:241–247
Demski LS, Northcutt RG (1983) The terminal nerve: a new chemosensory system in vertebrates? Science 220:435–437
Ebbesson SOE (1972) A proposal for a common nomenclature for some optic nuclei in vertebrates and the evidence for a common origin of two such cell groups. Brain Behav Evol 6:75–91
Ebbesson SOE, Meyer DL (1981) Efferents to the retina have multiple sources in teleost fish. Science 214:924–926
Ebbesson SOE, Meyer DL (1989) Retinopetal cells exist in the optic tectum of steelhead trout. Neurosci Lett 106:95–98
Ekström P (1984) Central neural connections of pineal organ and retina in the teleost Gasterosteus aculeatus L. J Comp Neurol 226:321–335
Ekström P, Honkanen T, Ebbesson SOE (1988) FMRF amide-like immunoreactive neurons of the nervus terminalis of teleosts innervate both retina and pineal organ. Brain Res 460:68–75
Fujita I, Satou M, Ueda K (1985) Ganglion cells of the terminal nerve: morphology and electrophysiology. Brain Res 335:148–152
Greenwood PH, Rosen DE, Weitzman SH, Myers GS (1966) Phyletic studies of teleostean fishes, with a provisional classification of living forms. Bull Am Mus Nat History 131:339–455
Grober MS, Bass AH, Buret G, Marchaterre MA, Segil N, Scholy K, Hodgason T (1987) The nervus terminalis ganglion in Anguilla rostrata: an immunocytochemical and HRP histochemical analysis. Brain Res 436:148–152
Khanna SS, Singh HR (1966) Morphology of the teleostean brain in relation to feeding habits. Proc Nat Acad Sci India 36:306–316
Klüver H, Barrera E (1953) A method for the combined staining of cells and fibres in the nervous system. J Neuropathol Exp Neurol 12:400–403
Lauder GV, Liem K (1983) Patterns of diversity and evolution in ray-finned fishes. In: Davis RE, Northcutt RG (eds) Fish neurobiology. University of Michigan Press, Ann Arbor, pp 1–24
Matsutani S, Uchiyama H, Ito H (1986) Cytoarchitecture, synaptic organization and fibre connections of the nucleus olfactoretinalis in a teleost (Navodon modestus). Brain Res 373:126–138
Meyer DL (1989) Retinopetal neurons in the diencephalon of juvenile Lobotes surinamensis (Teleostei). Neurosci Lett 107:51–55
Meyer DL, Ebbesson SOE (1981) Retinofugal and retinopetal connections in the upside-down catfish (Synodontis nigriventris). Cell Tissue Res 218:389–401
Meyer DL, Fiebig E, Ebbesson SOE (1981) A note on the reciprocal connections between the retina and the brain in the puffer fish (Tetradon fluviatilis). Neurosci Lett 23:111–115
Münz H, Claas B (1981) Centrifugal innervation of the retina in cichlid and poecilid fishes. A horseradish peroxidase study. Neurosci Lett 22:223–226
Münz H, Stumpf WE, Jennes L (1981) LHRH systems in the brain of platyfish. Brain Res 221:1–13
Münz H, Claas B, Stumpf WE, Jennes L (1982) Centrifugal innervation of the retina by luteinizing hormone releasing hormone (LH-RH)-immunoreactive telencephalic neurons in the teleostean fishes. Cell Tissue Res 222:313–323
Northcutt RG, Wullimann MF (1988) The visual system in teleost fishes: morphological patterns and trends. In: Atema J, Fay RR, Popper AN, Tavolga WN (eds) Sensory biology of aquatic animals. Springer, Berlin Heidelberg New York, pp 515–552
Oka Y, Munro AS, Lam TJ (1986) Retinopetal projections from a subpopulation of ganglion cells of the nervus terminalis in the dwarf grourami (Colisa lalia). Brain Res 367:341–345
Peyrichoux J, Weidner C, Repérant J, Miceli D (1977) An experimental study of the visual system of cyprinid fish using the HRP method. Brain Res 130:531–537
Prasada Rao PD (1967) Studies on the structural variations in the brain of teleosts and their significance. Acta Anat 68:379–398
Repérant J, Miceli D, Vesselkin NP, Molotchmikoff S (1989) The centrifugal visual system of vertebrates: a century-old search reviewed. Int Rev Cytol 118:115–171
Rusoff AC, Hapner SJ (1990) Organization of retinopetal axons in the optic nerve of cichlid fish, Herotilapia multispinosa. J Comp Neurol 294:418–430
Schmidt JT (1979) The laminar organization of optic fibres in the tectum of goldfish. Proc R Soc Lond (Biol) 205:287–306
Singh HR, Khanna SS (1970) Cytoarchitecture and fibre connections of the optic tectum of some teleosts. Zool Beitr 16:128–140
Springer AD (1983) Centrifugal innervation of goldfish retina from ganglion cells of the nervus terminalis. J Comp Neurol 214:404–415
Springer AD, Gaffney JS (1981) Retinal projections in the goldfish: a study using cobaltous lysine. J Comp Neurol 203:401–424
Springer AD, Mednick AS (1985) Retinofugal and retinopetal projections in the cichlid fish Astronotus ocellatus. J Comp Neurol 236:179–196
Springer AD, Prokosch JH (1982) Surgical and intensification procedures for defining visual pathways with cobaltous-lysine. J Histochem Cytochem 30:1235–1242
Stell WK, Walker SE, Chohan KS, Ball AK (1984) The goldfish nervus terminalis: a luteinizing hormone-releasing hormone and molluscan cardioexcitatory peptide immunoreactive olfactoretinal pathway. Proc Natl Acad Sci USA 81:940–944
Stell WK, Walker SE, Ball AK (1987) Functional-anatomical studies on the terminal nerve projection to the retina of bony fishes. Ann NY Acad Sci 519:80–96
Striedter GF, Northcutt RG (1989) Two distinct visual pathways through the superficial pretectum in a percomorph teleost. J Comp Neurol 283:342–354
Tesch FW (1977) The Eel. Chapman and Hall, London, pp 434
Uchiyama H (1989) Centrifugal pathways to the retina: influence of the optic tectum: a review. Vis Neurosci 3:183–206
Uchiyama H, Ito H (1984) Fibre connections and synaptic organization of the preoptic retinopetal nucleus in the filefish (Balistidae, Teleostei). Brain Res 298:11–24
Uchiyama H, Sakamoto N, Ito H (1981) A retinopetal nucleus in the preoptic area in a teleost, Navodon modestus. Brain Res 222:119–124
Uchiyama H, Reh TA, Stell WK (1988) Immunocytochemical and morphological evidence for retinopetal projections in anuran amphibians. J Comp Neurol 274:48–59
Vanegas H, Ito H (1983) Morphological aspects of the teleostean visual system: a review. Brain Res Rev 6:117–137
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prasada Rao, P.D., Kulkarni, A.P. Retinopetal neuronal system in the brain of an air-breathing teleost fish, Channa punctata . Cell Tissue Res 263, 385–394 (1991). https://doi.org/10.1007/BF00318780
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318780