Summary
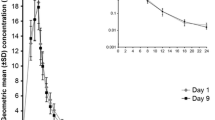
Ampicillin is often used in high oral doses due to its incomplete absorption. The objective of this study was to compare the bioavailability of bacampicillin, a new prodrug of ampicillin which is nearly completely absorbed, to that of high doses of oral ampicillin. Single oral doses of bacampicillin 400 mg or 800 mg and ampicillin 500 mg, 1 g, or 2 g were given to 12 male subjects according to a cross-over design after overnight fasting. The median time after administration till the peak serum level was 0.75 and 1.0 hour with bacampicillin 400 mg and 800 mg respectively, as compared to 1.5, 2.0 and 1.5 hours for the ampicillin doses. The mean of the individual peak serum level after bacampicillin 400 mg, was 7.7 mg/l, which is higher than that after ampicillin 500 mg, 5.2 mg/l, and about the same as that after ampicillin 1 g, 7.6 mg/l. A slightly higher mean peak level was seen after bacampicillin 800 mg, 12.2 mg/l, as compared to ampicillin 2 g, 10.5 mg/l. The absorption rate was highest with bacampicillin, for which 90% of the absorption had occurred 1 hour after administration as compared to 2.5 hours after administration of ampicillin. The relative bioavailability was about 2–3 times higher after bacampicillin as compared to ampicillin. The data imply dose-dependent absorption of oral ampicillin in high doses as there seems to be a less than proportional increase in AUC. The urinary recovery after bacampicillin was 68–75% as compared to 25–41% after ampicillin. Higher doses gave a lower percentage recovery and this phenomenon was more pronounced with ampicillin.
Zusammenfassung
Ampicillin wird aufgrund seiner unvollständigen oralen Resorption oft in hohen Dosen verwendet. Das Ziel der Studie war, die Bioverfügbarkeit von Bacampicillin, einem neuen Ester von Ampicillin, das fast vollständig resorbiert wird, mit der von Ampicillin in hohen oralen Dosen zu vergleichen. Orale Einzeldosen von 400 mg und 800 mg Bacampicillin und von 500 mg, 1 g und 2 g Ampicillin wurden an 12 männliche Versuchspersonen nach Fasten über Nacht im cross-over Verfahren verabreicht. Von der Einnahme von 400 mg bzw. 800 mg Bacampicillin bis zum Erreichen maximaler Serumspiegel vergingen im Mittel 0,75 bzw. 1,0 Stunden, bei Ampicillin in Dosen von 500 mg, 1 g und 2 g betrugen die Zeitintervalle im Mittel 1,5, 2,0 und 1,5 Stunden. Der Mittelwert der individuellen maximalen Serumspiegel betrug nach 400 mg Bacampicillin 7,7 mg/l, er war höher als nach 500 mg Ampicillin (5,2 mg/l) und entsprach etwa der mittleren maximalen Serumkonzentration von Ampicillin nach oraler Einzeldosis von 1 g (7,6 mg/l). Die maximalen Serumspiegel stiegen nach Einzeldosen von 800 mg Bacampicillin im Durchschnitt auf 12,2 mg/l, etwas höher also als der Mittelwert nach Gabe von 2 g Ampicillin, 10,5 mg/l. Bacampicillin wurde schneller resorbiert als Ampicillin, eine Resorptionsrate von 90% wurde bereits nach einer Stunde erreicht, bei Ampicillin erst nach zweieinhalb Stunden. Die relative Bioverfügbarkeit von Bacampicillin war 2–3mal größer als die von Ampicillin. Die gefundenen Daten deuten auf eine Dosis-abhängige veränderte Resorption von oralem Ampicillin in hohen Dosen hin, da die Vergrößerung der Fläche unter der Serumkonzentrationskurve geringer als proportional zur Dosis erfolgt. Es verändert sich also die Dosis-Absorptions-Relation. Die Urin-recovery betrug bei Bacampicillin 68–75%, bei Ampicillin 25–41%. Höhere Dosen ergaben eine niedrigere prozentuale recovery, dieses Phänomen war bei Ampicillin stärker ausgeprägt.
Similar content being viewed by others
Literature
Bolme, P., Dahlström, B., Diding, N. Å., Flink, O., Paalzow, L. Ampicillin: Comparison of bioavailability and pharmacokinetics after oral and intravenous administration of three brands. Eur. J. clin. Pharmacol. 10 (1976) 237–243.
Bodin, N.-O., Ekström, B., Forsgren, U., Jalar, L.-P., Magni, L., Ramsay, C.-H., Sjöberg, B. Bacampicillin: a new orally well-absorbed derivative of ampicillin. Antimicrob. Ag. Chemother. 8 (1975) 518–525.
Ekström, B., Forsgren, U., Jalar, L.-P., Magni, L., Sjöberg, B., Sjövall, J. Preclinical studies with bacampicillin-a new orally well absorbed prodrug of ampicillin. Drugs Exptl. Clin. Res. 3 (1977) 3–10.
Swahn, Å. On the absorption and metabolism of some penicillins in man. Dissertation, Stockholm, 1974.
Grove, D. C., Randall, W. A. Assay methods of antibiotics: A laboratory manual. Medical encyclopedia, Inc., New York, 1955.
Wagner, J. G. Fundamentals of clinical pharmacokinetics. Drug Intelligence Publications, Inc., Hamilton/Ill., 1975, p. 173.
Guenther, W. C. Analysis of variance. Prentice-Hall Inc., Englewood Cliffs/N. J., 1964.
Hollander, M., Wolfe, D. A. Nonparametric statistical methods. John Wiley and Sons, New York, 1973, p. 151–158.
Knudsen, E. T., Rolinson, G. N., Stevens, S. Absorption and excretion of “Penbritin”. Br. Med. J. 2 (1961) 198–200.
Hallander, H. O., Flodström, A., Sjövall, J. Pharmacological and clinical study of bacampicillin in acute peritonsillitis—a comparison with ampicillin. Antimicrob. Ag. Chemother. 11 (1977) 185–190.
Magni, L., Sjöberg, B., Sjövall, J., Wessman, J.: Clinical pharmacological studies with bacampicillin. In:Williams, J. D., Geddes, A. M.: Chemotherapy, Vol. 5: Penicillins and cephalosporins. Plenum Press, New York, p. 109–114.
Eriksson, G. Oral ampicillin in uncomplicated gonorrhoea. IV. Comparison of pharmacological and clinical results. Acta Derm. Venereol. (Stockholm) 51 (1971) 467–475.
Bergan, T. Pharmacokinetic comparison of oral bacampicillin and parenteral ampicillin. Antimicrob. Ag. Chemother. 13 (1978) 971–974.
MacLeod, C., Rabin, H., Ogilvie, R., Ruedy, J., Caron, M., Zarowny, D., Davies, R. O. Practical concepts of drug absorption, distribution and loss. Can. Med. Assoc. J. 111 (1974) 341–346.
Sjövall, J., Magni, L., Bergan, T. Pharmacokinetics of bacampicillin compared with those of ampicillin, pivampicillin, and amoxycillin. Antimicrob. Ag. Chemother. 13 (1978) 90–96.
Kunst, M. W., Mattie, H. Absorption of pivampicillin in post-operative patients. Antimicrob. Ag. Chemother. 8 (1975) 11–14.
Swahn, Å. Gastrointestinal absorption and metabolism of two35S-labelled ampicillin esters. Eur. J. clin. Pharmacol. 9 (1976) 299–306.
Swahn, Å. On the absorption and metabolism of35S-ampicillin. Eur. J. clin. Pharmacol. 9 (1975) 117–124.
Barza, M., Weinstein, L. Penetration of antibiotics into fibrin loci in vivo. I. Comparison of penetration of ampicillin into fibrin clots, abscesses, and “interstitial fluid”. J. Infect. Dis. 129 (1974) 59–65.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Magni, L., Sjövall, J. & Syvälahti, E. Comparative clinical pharmacology of bacampicillin and high oral doses of ampicillin. Infection 6, 283–289 (1978). https://doi.org/10.1007/BF01641988
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01641988