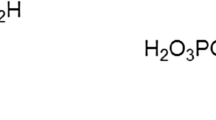Summary
Magnesium (Mg) is a conspicuous constituent of hard tissues but its possible role in biomineralization is poorly understood. It is possible that Mg2+ adsorbed onto bioapatites may contribute to the modulation of crystal growth as such inhibitory activity has been reported for synthetic apatites. The present study was undertaken to determine the adsorption isotherms of Mg ions onto synthetic apatites and biominerals in tooth and bone tissues in the presence of other ions of natural occurrence. Synthetic crystals used as adsorbents were hydroxyapatite and, as a better prototype for the biomineral, Mg-containing carbonatoapatite. Human enamel and dentin materials were obtained from extracted, caries-free, permanent teeth. Porcine dentin materials at two developmental stages were obtained from erupted deciduous and unerupted permanent teeth of a 6-month-old slaughtered piglet. Porcine bone was obtained from the cortical portion of the mandible of the same animal. All biomineral samples were pulverized and then treated by plasma ashing (deproteination) at about 60°C. Each of the powdered samples was equilibrated in solutions containing various initial concentrations of Mg2+, Ca2+, and Na+ (or K+) as nitrate salts. Following equilibration, concentrations (and activities) of magnesium and calcium ions in the experimental solution were determined. The pH values of the equilibrium solutions were in the range of 6.2–6.5. Experimental data of the Mg adsorption onto hydroxyapatite were interpreted on the basis of a Langmuir-type model for binary systems assuming competition of Mg2+ and Ca2+ for the same adsorption sites on the crystal surfaces of the apatites. According to this model, the adsorbed Mg is expressed as a function of the ionic activity ratio (Mg2+)/(Ca2+) in the equilibrium solution. The model contains two parameters, the adsorption selectivity constant Ks and the maximum number of adsorption sites N (μmol/g). The numerical values of Ks were similar for all adsorbents used (synthetic and biological) and indicated the preferential adsorption of Ca2+ probably due to spacial restrictions extending to the very surface of the crystals. The initial level of Mg2+ in the surface pool was different in the various biominerals, probably reflecting the composition of fluid in which the biominerals were formed. Whereas the surface pool of Mg of human enamel was marginal, only 5% of the total Mg, significant fractions of the total Mg in human and porcine dentins (about 20–30%), and porcine bone (about 40%) existed on the crystal surfaces. There were significant differences in the total Mg and the value of the parameter N between young (unerupted) and mature (erupted) dentin minerals. It was ascertained that the occupancy of adsorption sites by Mg ions became greater with maturation of the dentin tissues. The overall results suggest that the Mg-mineral interaction in tooth and bone tissues may be a highly tissue-specific process, presumably reflecting differences in fluid composition (particularly Ca and Mg activities) responsible for biomineralization.
Similar content being viewed by others
References
Rufenacht HS, Fleisch H (1984) Measurement of inhibitors of calcium phosphate precipitation in plasma ultrafiltrate. Am J Physiol 246:F648-F655
Meyer JL, Fleisch H (1984) Calcification inhibitors in rat and human serum and plasma. Biochim Biophys Acta 799:115–121
Wilson JWL, Werness PG, Smith LH (1985) Inhibitors of crystal growth of hydroxyapatite: a constant composition approach. J Urology 134:1255–1258
Amjad Z, Koutsoukos PG, Nancollas GH (1984) The crystallization of hydroxyapatites and fluorapatites in the presence of magnesium ions. J Colloid Interface Sci 101:250–256
Feenstra TP, Hop J, de Bruyn PL (1981) The influence of small amounts of magnesium on the formation of calcium phosphates in moderately supersaturated solutions. J Colloid Interface Sci 83:583–588
Moreno EC, Aoba T, Margolis HC (1987) Pyrophosphate adsorption onto hydroxyapatite and its inhibition of crystal growth. Compend Contin Educ Dent 8:S256-S266
de Boer JH (1953) The dynamical character of adsorption. Clarendon Press, Oxford
Wuthier RE (1977) Electrolytes of isolated epiphyseal chondrocytes, matrix vesicles, and extracellular fluid. Calcif Tissue Res 23:125–133
Aoba T, Moreno EC (1987) The enamel fluid in the early secretory stage of porcine amelogenesis. Chemical composition and saturation with respect to enamel mineral. Calcif Tissue Int 41:86–94
Larsson PA, Howell DS, Pita JC, Blanco LN (1988) Aspiration and characterization of predentin fluid in developing rat teeth by means of a micropuncture and micro-analytical technique. J Dent Res 67:870–875
Bachra BN, Trautz OR, Simon SL (1965) Precipitation of calcium carbonates and phosphates. III. The effect of magnesium and fluoride ions on the spontaneous precipitation of calcium carbonates and phosphates. Arch Oral Biol 10:731–738
Blumenthal NC, Betts F, Posner AS (1977) Stabilization of amorphous calcium phosphate by Mg and ATP. Calcif Tissue Res 23:245–250
Shimoda S, Aoba T, Moreno EC, Miake Y (1990) Effect of solution composition on morphological and structural features of carbonated calcium apatites. J Dent Res 69:1731–1740
Moreno EC, Aoba T (1990) Comparative solubility of human dental enamel, dentin, and hydroxyapatite. Calcif Tissue Int 49:6–13
Aoba T, Moreno EC (1990) Changes in the nature and composition of enamel mineral during porcine amelogenesis. Calcif Tissue Int 47:356–364
Vogel AJ (1961) Quantitative inorganic analysis, 3rd ed. John Wiley & Sons, New York, p 810
Kreling JR, DeZwaan J (1986) Ion chromatographic procedure for bicarbonate determination in biological fluids. Anal Chem 58:3028–3031
Moreno EC, Margolis HC (1988) Composition of human plague fluid. J Dent Res 67:1181–1189
Moreno EC, Kresak M, Kane JJ, Hay DI (1987) Adsorption of proteins, peptides, and organic acids from binary mixtures onto hydroxyapatite. Langumir 3:511–519
Monk CB (1961) Electrolytic dissociation. Academic Press, London, New York, p 271
Neuman WF, Mulryan BJ (1971) Synthetic hydroxyapatite crystals. IV. Magnesium incorporation. Calcif Tissue Res 7:133–138
Terpstra RA, Driessens FC (1986) Magnesium in tooth enamel and synthetic apatites. Calcif Tissue Int 39:348–354
LeGeros RZ (1984) Incorporation of magnesium in synthetic and in biological apatites. In: Tooth enamel IV. pp 32–36
Casciani FS, Etz ES, Newbury DE, Doty SB (1979) Raman microprobe studies of two mineralizing tissues: enamel of the rat incisor and the embryonic chick tibia. Scanning Electron Microsc II:383–390
Driessens FCM, Verbeeck RMH (1985) Dolomite as a possible magnesium-containing phase in human tooth enamel. Calcif Tissue Int 37:376–380
Quint P, Althoff J, Hohling HJ, Boyde A, Laabs WA (1980) Characteristic molar ratios of magnesium, carbon dioxide, calcium and phosphorus in the mineralizing fracture callus and predentine. Calcif Tissue Int 32:257–261
Robinson C, Weatherell JA (1989): Magnesium in bone and tooth. In: Metal ions in biological systems vol 26: magnesium and its role in biology, nutrition and physiology. Marcel Dekkar Inc. New York, pp 489–504
Steinfort J, Driessens FCM, Heijligers HJM, Beertsen W (1991) The distribution of magnesium in developing rat incisor dentin. J Dent Res 70:187–191
Linde A, Lundgren T (1990) Calcium transport in dentinogenesis. J Biol Buccale 18:155–160
Moreno EC, Varughese K, Hay DI (1979) Effect of human salivary proteins on the precipitation kinetics of calcium phosphate. Calcif Tissue Int 28:7–16
Aoba T, Moreno EC, Hay DI (1984) Inhibition of apatite crystal growth by the amino-terminal segment of human salivary acidic proline-rich proteins. Calcif Tissue Int 36:651–658
Aoba T, Fukae M, Tanabe T, Shimizu M, Moreno EC (1987) Selective adsorption of porcine amelogenins onto hydroxyapatite and their inhibitory activity on seeded crystal growth of hydroxyapatite. Calcif Tissue Int 41:281–289
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aoba, T., Moreno, E.C. & Shimoda, S. Competitive adsorption of magnesium and calcium ions onto synthetic and biological apatites. Calcif Tissue Int 51, 143–150 (1992). https://doi.org/10.1007/BF00298503
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00298503