Summary
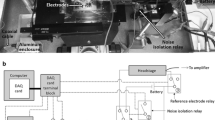
We have developed a miniature silver-silver chloride electrode. The outer diameter of the electrodes averaged 22 μm and the input resistance 8.8 kΩ. Since the core of the electrode is a glass fiber, the problem of the extreme malleability of a small diameter silver fiber is circumvented. The properties of the electrode permit us to insert it into short (600 μm) fragments of the amphibian collecting duct while they are being perfusedin vitro. The passage of currents in the range of 0 to 6×10−8 amperes allowed us to voltage clamp the nephron fragment between +20 and −20 mV. The current-voltage plots are linear over this range. Two lines of evidence suggest that the voltage clamp is homogeneous. First, the voltage measured at the perfusion end during a voltage-clamp experiment of the tubule is not significantly different from that measured at the collecting end. Secondly, the specific resistance of collecting ducts estimated from the “core conductor analysis” is 3.3±0.8×104 Ω cm, a value not significantly different from that computed from the current-voltage plots as determined with the Ag−AgCl electrode, 3.0±0.5×104 Ω cm. This method permits precise control of both the ionic and electrical gradients across fragments of the amphibian collecting duct.
Similar content being viewed by others
References
Augustus, J., Bijman, J. 1977. An electrode which simultaneously passes current and measures potential and is Cl− sensitive.Pfluegers Arch. 370:87–91
Bourdeau, J.E., Burg, M.S. 1979. Voltage dependence of calcium transport in the thick ascending limb of Henle's loop.Am. J. Physiol. 234:F357-F364
Burg, M.B., Grantham, J., Abramow, M., Orlogg, J. 1966. Preparation and study of fragments of single rabbit nephrons.Am. J. Physiol. 210:1293–1298
Chase, S.W. 1923. The mesonephros and urogenital ducts ofNecturus maculosus, Rafinesque.J. Morphol. 37:457–531
Eigler, F.W. 1961. Short-circuit current measurements in proximal tubule ofNecturus kidney.Am. J. Physiol. 201:157–163
Garcia-Filho, E., Malnic, G., Giebisch, G. 1980. Effects of changes in electrical potential difference on tubular potassium transport.Am. J. Physiol. 238:F235-F246
Grantham, J.J., Burg, M.B., Orloff, J. 1970. The nature of transtubular Na+ and K+ transport in isolated rabbit renal collecting tubules.J. Clin. Invest. 49:1815–1826
Hegel, U., Boulpaep, E.L. 1975. Studies of electrical impedance of kidney proximal tubular epithelium inNecturus. 6th Int. Congr. Nephrol. Firenze. (Abstr. 45)
Helman, S.I. 1972. Determination of electrical resistance of the isolated cortical collecting tubule and its possible anatomical location.Yale J. Biol. Med. 45:339–345
Helman, S.I., Grantham, J.J., Burg, M.B. 1971. Effect of vasopressin on electrical resistance of renal cortical collecting tubules.Am. J. Physiol. 220:1825–1832
Koefoed-Johnsen, V., Ussing, H.H. 1958. The nature of the frog skin potential.Acta Physiol. Scand. 42:298–308
Marmont, G. 1949. Studies on the axon membrane.J. Cell. Comp. Physiol. 34:351–382
Pasto, D.J., Johnson, C.R. 1969. Tollen's test for Aldehydes.In: Organic Structure Determination. pp 383–384. Prentice-Hall, Englewood, New Jersey
Spring, K.R. 1973. Current induced voltage transient inNecturus proximal tubule under voltage clamp.J. Membrane Biol. 13:299–322
Spring, K.R., Paganelli, C.V. 1972. Sodium flux inNecturus proximal tubule under voltage clamp.J. Gen. Physiol. 60:181–201
Stoner, L.C. 1977. Isolated perfused amphibian renal tubules: The diluting segment.Am. J. Physiol. 233:F438-F444
Stoner, L., Burg, M., Orloff, J. 1974. Ion transport in cortical collecting tubule: Effect of amiloride.Am. J. Physiol. 227:453–459
Ussing, H.H. 1949. The active ion transport through the isolated frog skin in the light of tracer studies.Acta Physiol. Scand. 17:1–37
Ussing, H.H., Zerahn, D. 1951. Active transport of sodium as the source of electric current in the short circuited isolated frog skin.Acta Physiol. Scand. 23:110–127
Windhager, E.E., Giebisch, G. 1961. Comparison of short circuit current and net water movement in single perfused proximal tubules of rat kidneys.Nature (London) 191:1205
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Delaney, R., Stoner, L.C. Miniature Ag−AgCl electrode for voltage clamping of theAmbystoma collecting duct. J. Membrain Biol. 64, 45–53 (1982). https://doi.org/10.1007/BF01870767
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01870767