Summary
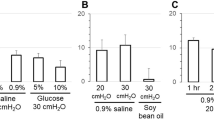
Phenamil, an analog of amiloride, has previously been shown to bind specifically to sodium channels in toad bladder (J.L. Garvin et al.,J. Membrane Biol. 87:45–54, 1985). In this paper,3H-phenamil was used to measure sodium channel density in both isolated epithelial cells and intact bladders. From the specific binding to intact bladders, a channel density of 455±102 channels/μm2 was calculated. No correlation between specific binding and the magnitude of irreversible inhibition of shortcircuit current was found. Pretreatment of intact bladders with 1 mg/ml trypsin reduced specific binding to isolated cells by 82±5%. In isolated cells, neither aldosterone nor vasopressin had any significant effect on specific phenamil binding. It is inferred that phenamil binds to both open and closed channels which may be either in the mucosal membrane or in the submembrane space. Finally, and rather surprisingly, we found that3H-phenamil binds irreversibly to the basolateral membrane at concentrations as low as 4×10−7 m. Therefore, care must be used in interpreting binding studies with amiloride or its analog at such concentrations.
Similar content being viewed by others
References
Abrahamcheck, F.J., Van Driesche, W., Helman, S.I. 1985. Autoregulation of apical membrane Na+ permeability of tight epithelia. Noise analysis with amiloride and CGSS 4270.J. Gen. Physiol. 85:555–582
Benjamin, W.B., Singer, I. 1974. Aldosterone-induced proteins in toad urinary bladder.Science 186:269–272
Benos, D.J. 1982. Amiloride: A molecular probe of sodium transport in tussues and cells.Am. J. Physiol. 242:C131-C145
Benos, D.J., Reyes, J., Shoemaker, D.G. 1983. Amiloride fluxes across erythrocyte membranes.Biochim. Biophys. Acta 734:99–104
Benos, D.J., Whatthey, J.W.H. 1981. Inferences on the nature of the apical sodium entry site in frog skin epithelium.J. Pharmacol. Exp. Ther. 219:481–488
Cuthbert, A.W., Okpako, D., Shum, W.K. 1974. Aldosterone, moulting, and the number of sodium channels in frog skin.Brit. J. Pharmacol. 51:128P
Cuthbert, A.W., Shum, W.K. 1975. Effects of vasopressin and aldosterone on amiloride binding in toad bladder epithelial cells.Proc. R. Soc. London B 189:543–575
Edelman, I.S., Bogoroch, R., Porter, G.A. 1963. On the mechanism of action of aldosterone on sodium transport: The role of protein synthesis.Proc. Natl. Acad. Sci. USA 50:1169–1177
Garty, H., Edelman, I.S. 1983. Amiloride-sensitive trypsinization of apical sodium channels.J. Gen. Physiol. 81:785–803
Garvin, J.L., Simon, S.A., Cragoe, E.J., Jr., Mandel, L.J. 1985. Phenamil: An irreversible inhibitor of sodium channels in the toad urinary bladder.J. Membrane Biol. 87:45–54
Leaf, A., Macknight, A.D.C. 1972. The site of the aldosteroneinduced stimulation of sodium transport.J. Steroid Biochem. 3:237–245
Li, J.H.-Y., Lindemann, B. 1983. Chemical stimulation of Na transport through amiloride-blockable channels of frog skin epithelium.J. Membrane Biol. 75:179–192
Li, J.H.-Y., Palmer, L.G., Edelman, I.S., Lindemann, B. 1982. The role of sodium-channel density in the natriferic response of the toad urinary bladder to an antidiuretic hormone.J. Membrane Biol. 64:77–89
Lindemann, B., Van Driessche, W. 1977. Sodium-specific membrane channels of frog skin are pores: Current fluctuations reveal high turnover.Science 195:292–294
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. 1951. Protein measurement with the Folin phenol reagent.J. Biol. Chem. 193:265–275
Meyer, D.J., Yancy, S.B., Revel, J.-P. 1981. Intercellular communication in normal and regenerating rat liver: A quantitative analysis.J. Cell Biol. 91:505–523
Palmer, L.G., Li, J.H.-Y., Lindemann, B., Edelman, I.S. 1982. Aldosterone control of the density of sodium channels in the toad urinary bladder.J. Membrane Biol. 64:91–102
Sariban-Sohraby, S., Burg, M.G., Turner, R.J. 1983. Apical sodium uptake in the toad kidney epithelial cell line A6.Am. J. Physiol. 245:C167-C171
Stetson, D.L., Lewis, S.A., Alles, W., Wade, J.B. 1982. Evaluation by capacitance measurements of antidiuretic hormone induced membrane area changes in toad bladder.Biochim. Biophys. Acta 689:267–274
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garvin, J.L., Simon, S.A., Cragoe, E.J. et al. Binding of3H-phenamil, an irreversible amiloride analog, to toad urinary bladder: effects of aldosterone and vasopressin. J. Membrain Biol. 90, 107–113 (1986). https://doi.org/10.1007/BF01869928
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01869928