Abstract
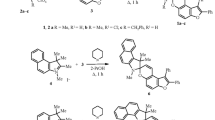
Starting from (+) (2R) methyl 5′-ethyl-2,2′-spirobiindane-5-carboxylate of known enantiomeric purity 79 optically active, configurationally correlated 5,5′,6′-trisubstituted 2,2′-spirobiindanes (2–7) were prepared for the purpose of testing a “shortened polynomal Ansatz” for chirality functions. Their optical rotations and1H-nmr spectra are reported.
In this context several 6-substituted 5-ethylindanes (1) were prepared as model compounds for synthetic transformations.
Similar content being viewed by others
Literatur
7.Mitt:E. Langer, H. Lehner, H. Neudeck undK. Schlögl, Mh. Chem.109, 987 (1978).
A. Meyer, H. Neudeck undK. Schlögl, Chem. Ber.110, 1403 (1977).
H. Neudeck undK. Schlögl, Chem. Ber.110, 2624 (1977).
E. Ruch, W. Runge undG. Kresze, Angew. Chem.85, 10 (1973); Intern. Ed. Engl.12, 20 (1973); siehe auchE. Ruch undA. Schönhofer, Theor. Chim. Acta,19, 225, (1970).
H. Neudeck, B. Richter undK. Schlögl, Mh. Chem., im Druck.
K. Kindler undT. Li, Ber. dtsch. chem. Ges.74, 321 (1941).
Ng. Ph. Buu-Hoi undNg. D. Xuong, J. Chem. Soc.,1952, 2225 (Ohne nähere Angaben).
R. Pfleger undK. Bauer, Chem. Ber.90, 1500 (1957).
A. Rieche, H. Gross undE. Höft, Chem. Ber.93 88 (1960).
Vgl.A. Fatiadi, Synthesis1976, 65.
M. Avaro, J. Levisalles undU. H. Rudler, Chem. Commun.,1969, 445R. F. Smith undL. E. Walker, J. Org. Chem.,27, 4372 (1962).
P. A. S. Smith, J. Amer. Chem. Soc.70, 320 (1948).
R. T. Canley, J. Org. Chem.23, 1330 (1958).
L. Sihlbom, Acta Chem. Scand.8, 529 (1954).
E. J. Corey, N. W. Gilman undB. E. Ganem, J. Amer. Chem. Soc.90, 5616 (1968).
M. Shamma undH. R. Rodriguez, Tetrahedron24, 6583 (1968).
E. F. Pratt undJ. F. van de Castle, J. Org. Chem.26, 2973 (1961).
K. Nakagawa, R. Konaka undT. Nakata, J. Org. Chem.27, 1597 (1962).
L. F. Fieser undS. Rajagopalan, J. Amer. Chem. Soc.71, 3938 (1949).
J. D. Albright undL. Goldman, J. Amer. Chem. Soc.87, 4214 (1965).
K. E. Pfitzner undJ. G. Moffatt, J. Amer. Chem. Soc.87, 5670 (1965).
Vgl.H. C. Brown, Ch. P. Garg undK. T. Liu, J. Org. Chem.36, 387 (1971).
Vgl.R. Ratcliffe undR. Rodehurst, J. Org. Chem.35, 4000 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neudeck, H., Schlögl, K. Optisch aktive, aromatische Spirane, 8. Mitt.: Darstellung optisch aktiver, 5, 5′, 6′-trisubstituierter 2,2′-Spirobiindane bekannter Chiralität und enantiomerer Reinheit. Monatshefte für Chemie 110, 541–565 (1979). https://doi.org/10.1007/BF00938359
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00938359