Abstract
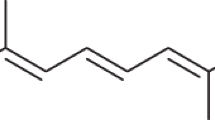
DURING some experiments with benzene extracts of various fruits, it was observed that the calcium hydroxide chromatogram frequently, but not in every case, shows a rather brownish orange layer below the lycopene zone. Subsequent experiments have proved that this behaviour of carefully purified lycopene depends on the time which elapses between the dissolution and the adsorption analysis. A fresh solution forms a uniform chromatogram, but if the liquid has been kept at 20° for 1–2 days, the new polyene (“neo-lycopene”) is observed ; it then forms a very distinctly separated zone in the chromatogram developed with benzene and light petroleum (3: 1). The pigment content of this layer reached 3 per cent of the total lycopene in experiments of 24 hours duration; the absorption maxima are 511, 479·5, 450 mµ in benzene and 498·5, 468, 440 mµ in petroleum ether. When the benzene solution is kept at room temperature, neo-lycopene is partly transformed into lycopene (or into a very similar dye which cannot be separated from lycopene in the column).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gillam and El Ridi, NATURE, 136, 914 (1935); Biochem. J., 30, 1735 (1936); Gillam, El Ridi and Kon, Biochem. J., 31, 1606 (1937).
Zechmeister and Cholnoky, Liebigs Ann., 530, 291 (1937).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ZECHMEISTER, L., TUZSON, P. Spontaneous Isomerization of Lycopene. Nature 141, 249–250 (1938). https://doi.org/10.1038/141249a0
Published:
Issue Date:
DOI: https://doi.org/10.1038/141249a0
This article is cited by
-
Rat intestinal homogenate and pancreatic juice can induce the Z-isomerization of all-E-lycopene in vitro
Scientific Reports (2020)
-
Some stereochemical aspects of polyenes
Experientia (1954)
-
Effect of Solvents on the Absorption Spectrum of Vitamin A
Nature (1938)
-
Action of Molybdenum in Nutrition of Milking Cattle
Nature (1938)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.