Abstract
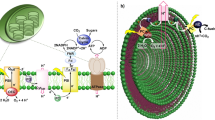
THE creation of artificial solar energy conversion and storage systems that mimic the photosynthetic pathway has evoked great interest in recent years1. One approach to the photolysis of water involves the mediation of two photosystems in the generation of reduced and oxidised species that are the active components in the decomposition of water2,3. However, homogeneous (aqueous) solutions of these components suffer from the basic limitation that the reducing and oxidising agents can react with each other and thus no net reaction can be observed. Several kinds of synthetic ‘photosynthetic membranes’ have been suggested as a means of separating the two redox units and overcoming these fundamental difficulties3. Recently, photo-sensitised electron transfer across vesicle walls has been demonstrated and suggested as a means for generating oxidising and reducing agents in separate water compartments4. We report here a photochemical electron transfer across the interface of a water-in-toluene microemulsion, and propose this system as a model for the separation of oxidised and reduced species. It is well known that surfactant molecules aggregate to reversed micelles in organic solvents5. Reversed micelles entrap water to form ‘water pools’ in a continuous oil phase. The proposed general model for the compartmentalisation of two water phases and its utilisation in the photolysis of water is shown in Fig. 1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Balzani, V., Maggi, L., Manfrin, M. F., Bolleta, F. & Gleria, M. Science, 189, 852–856 (1975).
Bolton, J. R. Science 202, 705–711 (1978).
Calvin, M. Acc. chem. Res. 11, 369–374 (1978).
Ford, W. E., Otvos, J. W. & Calvin, M. Nature 274, 507–508 (1978).
Fendler, J. H. & Fendler, F. J. Catalysis in Micelles and Macromolecular Systems (Academic, New York, 1975).
Steckman, E. & Kuwana, T. Ber. Bunsenges 78, 253–259 (1974).
Lim, Y. Y. & Fendler, J. H. J. Am. chem. Soc. 100, 7490–7494 (1978).
Van Houten, J. & Watts, R. J. J. Am. chem. Soc. 98, 4853–4858 (1976).
Nakamura, K., Ohno, A., Yasui, S. & Oka, S. Tetrahedron Lett. 4815–4818 (1978).
Newbold, B. T. in The Chemistry of the Hydrazo, Azo and Azoxy Groups (ed. Patai, S.) Chap. 15 (Wiley, New York, 1975).
Krasna, A. I. Photochem. Photobiol. 29, 267–276 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WILLNER, I., FORD, W., OTVOS, J. et al. Photoinduced electron transfer across a water–oil boundary as a model for redox reaction separation. Nature 280, 823–824 (1979). https://doi.org/10.1038/280823a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/280823a0
This article is cited by
-
Visible-light-assisted multimechanism design for one-step engineering tough hydrogels in seconds
Nature Communications (2020)
-
Dynamics of electron transfer in photosystem II
Cell Biochemistry and Biophysics (2007)
-
Forty years of photosynthesis and related activities
Photosynthesis Research (1989)
-
Redox potentials of alkylviologens incorporated into the membranes of lipid vesicles
Bulletin of the Academy of Sciences of the USSR Division of Chemical Science (1986)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.