Abstract
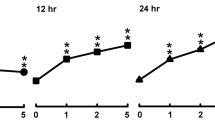
Prostaglandin E2 (PGE2) at concentrations more than 1×10−8 M markedly suppressed the cell proliferation and release of soluble molecules of interleukin-2 receptor (sIL-2R), CD4 (sCD4) and CD8 (sCD8) from phytohemagglutinin (PHA)-stimulated normal human mononuclear cells (MNC) in a dose-related manner. To further elucidate the subcellular mechanism of the inhibitory effect of PGE2 on PHA-stimulated MNC, intracellular concentration of glutathione (GSH) in PHA-stimulated MNC was sequentially measured from day 1 to day 3 by enzymic method. Furthermore, the effect of PGE2 on nuclear DNA including DNA strand breaks in alkali treatment and DNA fragmentation (apoptosis) of PHA-stimulated MNC were also measured. We found intracellular GSH levels were significantly decreased in the early stage of lymphocyte activation (day 1), but no evidence of increased DNA stand breaks or apoptotic process appeared in 3-day culture. In addition, butathione sulfoximine (a specific GSH inhibitor) and dibutyryl cyclic AMP also exhibited both proliferation inhibition and GSH-decreasing effect on PHA-stimulated MNC as well as PGE2. These results suggest that the immunosupressive effect of PGE2 is mediated by the decreased generation of intracellular GSH, but not by the increased DNA strand breaks or apoptotic mechanism in the cells.
Similar content being viewed by others
Abbreviations
- PGE2 :
-
prostaglandin E2
- GSH:
-
glutathione
- MNC:
-
mononuclear cell
- PHA:
-
phytohemagglutinin
- sIL-2R:
-
soluble interleukin-2 receptor
- sCD4:
-
soluble CD4 molecule
- sCD8:
-
soluble CD8 molecule
- ELISa:
-
enzyme-linked immunosorbent assay
- BSO:
-
dl-buthionine-[S,R]-sulfoximine
References
J. J. Ellner and P. J. Spagnuolo,Suppression of antigen-and mitogen-induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J. Immunol.123, 2689–2695 (1979).
J. S. Goodwin, A. D. Bankhurst and R. P. Messner,Suppression of human T-cell mitogenesis by prostaglandin. J. Exp. Med.146, 1719–1734 (1977).
N. D. Staite and G. S. Panayi,Regulation of human immunglobulin production in vitro by prostaglandin E 2. Clin. Exp. Immunol.49, 115–122 (1982).
N. J. Simkin, D. F. Jelinek and P. E. Lipsky,Inhibition of human B cell responsiveness by prostaglandin E 2. J. Immunol.138, 1074–1081 (1987).
S. L. Kunkel, R. C. Wiggins, S. W. Chensue and J. Larrick,Regulation of macrophage tumornecrosis factor production by prostgalandin E 2. Biochem. Biophys. Res. Commu.137, 404–410 (1986).
M. W. J. A. Fieren, C. M. van den Bemb G-J, S. Ben-Efraim and I. L. Bonta,Prostaglandin E 2 inhibits the release of tumor necrosis factor-α, rather than interleukin 1β, from human macrophage. Immunol. Lett.31, 85–90 (1991).
E. D. Anastassiou, F. Paliogianni, J. P. Balow, H. Yamada and D. T. Boumpas,Prsotaglandin E 2 and other cyclic AMP-elevating agents modulate IL-2 and IL-2R gene expression at multiple levels. J. Immunol.148, 2845–2852 (1992).
D. S. Snyder, D. I. Beller and E. R. Unanue,Prostaglandins modulate macrophage Ia expression. Nature299, 163–165 (1982).
C. S. Tripp, A. Wyche, E. R. Unanue and P. Needleman,The functional significance of the regulation of macrophage Ia expression by endogenous arachidonate metabolites in vitro. J. Immunol137, 3915–3920 (1986).
J. S. Goodwin and D. R. Webb,Regulation of the immune response by prostaglandins. Clin. Immunol. Immunopathol.15, 106–122 (1980).
E. Garaci, A. Mastino, T. Jezzi, C. Riccardi and C. Favalli,Effect of in vitro administration ofprostaglandins and interferon on natural killer activity and on B-16 melanoma growth in mice. Cell. Immunol.106, 43–52 (1987).
R. N. Spengler, M. L. Spengler, P. Licoln, D. G. Remick, R. M. Strieter and S. L. Kunkel,Dynamics of dibutyryl cyclic AMP and prostaglandin E 2-mediated suppression of lipopolysaccharide-induced tumor necrosis factor alpha gene expression. Infect. Immun.57, 2837–2841 (1989).
M. N. Elmasry, E. J. Fax, and R. R. Rich,Sequential effects of prostaglandins and interferon-γ on differentiation of CD8+suppressor cells. J. Immunol.139, 688–694 (1987).
A. B. Tilden and C. M. Balch,A comparison of PGE 2 effects on human suppressor cell function and on interleukin 2 function. J. Immunol.129, 2469–2473 (1982).
D. M. Brown, G. L. Warner, J. E. Ales-Martinez, D. W. Scott and R. P. Phipps,Prostaglandin E 2 induces apoptosis in immature normal and malignant B lymphocytes. Clin. Immunol. Immunopathol.63, 221–229 (1991).
A. H. Wyllie,Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature284, 555–556 (1980).
C. M. Fischman, M. C. Udey, M. Kurtz and H. J. Wedner,Inhibition of lectin-induced lymphocyte activation by 2-cyclohexene-1-one: decreased intracellular glutathione in hibits and early event in the activation sequence. J. Immunol.127, 2257–2262 (1981).
D. L. Hamilos and H. J. Wedner,The role of glutathione in lymphocyte activation. I. comparison of inhibitory effects of buthionine sulfoximine and 2-cyclohexene-1-one by nuclear size transformation. J. Immunol.135, 2740–2747 (1985).
J. P. Messner and D. A. Lawrence,Cell cycle progression of glutathione depleted human peripheral blood mononuclear cells is inhibited at S phase. J. Immunol.143, 1974–1981 (1989).
F. Tietze,Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem.27, 502–522 (1969).
H. C. Birnboim and J. J. Jevcak,Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res.41, 1889–1892 (1981).
D. P. Jones, D. J. McConkey, P. Nicotera and S. Orrenius,Calcium-activated DNA fragmentatin in rat liver nuclei. J. Biol. Chem.264, 6398–6403 (1989).
L. A. Rheins, L. Barnes, S. Amornsiripanitch, C. E. Collins, and J. J. Nordlund,Suppression of the cutaneous immune response following topical application of the prostaglandin PGE 2. Cell. Immunol.106, 33–42 (1987).
P. A. Thompson, D. F. Jelinek and P. E. Lipsky,Regulation of human B cell proliferation by prostaglandin E 2. J. Immunol.133, 2446–2453 (1984).
D. F. Jelinek, P. A. Thompson and P. E. Lipsky,Regulation of human B cell activation by prostaglandin E 2:suppression of the generation of immunoglobulin-secreting cells. J. Clin. Invest.75, 133–1349 (1985).
Y. Buchmuller-Rouiller, S. Betz-Corradin and J. Mauel,Differential effects of prostaglandins and macrophage activation induced by clacium ionophore A23187 or IFN-γ. J. Immunol.148, 1171–1175 (1992).
L. A. Rubin, C. C. Kurman, M. E. Fritz, W. E. Biddison, B. Boutin, R. Yarchoan and D. L. Nelson,Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J. Immunol.135, 3175–3177 (1985).
J. A. Symons, J. F. McCulloch, N. C. Wood and G. W. Duff,Soluble CD4 in patients with rheumatoid arthritis and osteoarthritis. Clin. Immunol. Immunopathol.60, 72–82 (1991).
B. E. Tomkinson, M. C. Brown, S. H. Ip, S. Carrabis and J. L. Sullivan,Soluble CD8 during T cell activation. J. Immunol.14, 2230–2236 (1989).
J. F. R. Kerr, A. H. Wyllie and A. R. Currie,Apoposis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer26, 239–257 (1972).
D. J. McConkey, P. Nicotera, P. Hartzell, G. Bellomo, A. H. Wyllie and S. Orrenius,Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca 2+ concentration. Arch. Biochem. Biophys.269, 365–370 (1989).
O. Janssen, S. Wesselborg, B. Heckl-Ostreicher, K. Pechhold, A. Bender, S. Schondelmaier, G. Moleenhauer and D. Kabelitz, T cell receptor/CD3-signaling induces death by apoptosis in human T cell receptor γδ+ T cells. J. Immunol.146, 35–39 (1991).
A. Zychlinsky, L. M. Zhen, C.-C. Liu and D.-E. Young,Cytolytic lymphocytes induce both apoptosis and necrosis in target cells. J. Immunol.146, 393–400 (1991).
D. Kabelitz, T. Pohl and K. Pechhod,Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol. Today14, 338–339 (1993).
M. V. Fanger, D. A. Hart, J. V. Wells and A. Nisonoff,Enhancement by reducing agents of the transformation of human and rabbit peripheral lymphocytes. J. Immunol.105, 1043–1045 (1970).
P. W. Reed,Glutathione and the hexose monophosphate shunt in phagocytozing and hydrogen peroxide-treated rat leukocytes. J. Biol Chem.244, 2459–2464 (1969).
C.-C. Ting, M. E. Hargrove, S.-M. Liang, C.-M. Ling and S. O. Sharrow,Dichotomy of glutathione regulation of the activation of resting and preactivated lymphocytes. Cell. Immunol.142, 40–53 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yu, C.L., Liu, C.L., Tsai, C.Y. et al. Prostaglandin E2 suppresses phytohemagglutin-induced immune responses of normal human monoculear cells by decreasing intracellular glutathione generation, but not due to increased DNA strand breaks or apoptosis. Agents and Actions 40, 191–199 (1993). https://doi.org/10.1007/BF01984061
Issue Date:
DOI: https://doi.org/10.1007/BF01984061