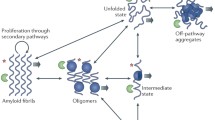
Abstract.
The physiological metabolism of proteins guarantees that different cellular compartments contain the appropriate concentration of proteins to perform their biological functions and, after a variable period of wear and tear, mediates their natural catabolism. The equilibrium between protein synthesis and catabolism ensures an effective turnover, but hereditary or acquired abnormalities of protein structure can provoke a premature loss of biological function, an accelerated catabolism and diseases caused by the loss of an irreplaceable function. In certain proteins, abnormal structure and metabolism are associated with a strong tendency to self-aggregation into a polymeric fibrillar structure, and in these cases the disease is not principally caused by the loss of an irreplaceable function but by the action of this new biological entity. Amyloid fibrils are an apparently inert, insoluble, mainly extracellular protein polymer that kills the cell without tissue necrosis but by activation of the apoptotic mechanism. We analyzed the data reported so far on the structural and functional properties of four prototypic proteins with well-known biological functions (lysozyme, transthyretin, β2-microglobulin and apolipoprotein AI) that are able to create amyloid fibrils under certain conditions, with the perspective of evaluating whether the achievement of biological function favors or inhibits the process of fibril formation. Furthermore, studying the biological functions carried out by amyloid fibrils reveals new types of protein-protein interactions in the transmission of messages to cells and may provide new ideas for effective therapeutic strategies.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 9 November 1998; received after revision 15 January 1999; accepted 15 January 1999
Rights and permissions
About this article
Cite this article
Bellotti, V., Mangione, P. & Stoppini, M. Biological activity and pathological implications of misfolded proteins. CMLS, Cell. Mol. Life Sci. 55, 977–991 (1999). https://doi.org/10.1007/s000180050348
Issue Date:
DOI: https://doi.org/10.1007/s000180050348