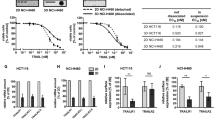
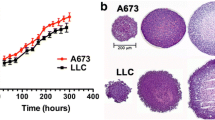
Abstract
Multicellular tumour spheroids (MCTS) are three-dimensional cell culture systems which are widely used in cancer research. They are characterized by an outer zone of proliferating cells, an inner region of differentiating quiescent cells and an area of so-called necrotic cell death in their centre. The exact cause of this cell death, a controversy for many years, was the aim of the present study. Our data show that cell death in the centre of MCTS of three colorectal adenocarcinoma cell lines (HRT-18, HT-29 and CX-2) was induced by apoptosis. Apoptotic cells were initially distributed at random but accumulated very quickly in the quiescent and central area at day 4–5, suggesting a time- rather than size-dependent synchronization of apoptosis parallel to the formation of the proliferation gradient in MCTS. To study mechanisms inducing apoptosis, the Fas-pathway was investigated. A cell--cell contact-dependent expression of CD95 was found in all MCTS. FasL was not detected in monolayer cultures, but was expressed in spheroids of HRT-18 and CX-2. We found that TNFα and TGFβ1 activated the CD95 pathway in all three cell lines. Since both TNF-α and TGF-β are known to be inducible by hypoxia in a variety of cell types, we suggest that these hypoxia-induced factors sensitize the CD95 pathway in the quiescent area of MCTS. Furthermore, a loss of the heat shock proteins 27, 32, 60, 73 and 90 was observed in the quiescent area of spheroids. This suggests that tumour cell differentiation in the inner region of MCTS may be an additional factor inducing apoptosis.
Similar content being viewed by others
References
Sutherland RM. Cells and environment interactions in tumor microregions: the multicell spheroid model. Science 1988; 240: 177-184.
Knuechel R, Feichtinger J, Recktenwald A, et al. Interactions between bladder tumor cells as tumor spheroids from the cell line J82 and human endothelial cells in vitro. J Urol 1988; 139: 640-645.
Mueller-Klieser W. Multicell spheroids: a review on cellular aggregates in cancer research. J Cancer Res Clin Onco 1987; 113: 101-122.
Carlsson J. A proliferation gradient in three-dimensional colonies of cultured human glioma cells. Int J Cancer 1977; 20: 129-136.
Freyer JP, Sutherland RM. Regulation of growth saturation and development of necrosis in EMT6/Ro multicellular spheroids by the glucose and oxygen supply. Cancer Res 1986; 46: 3504-3512.
Karbach U, Gerharz CD, Groebe K, Gabbert HE, Mueller-Klieser W. Rhabdomyosarcoma spheroids with central proliferation and differentiation. Cancer Res 1992; 52: 474-477.
Freyer JP, Sutherland RM. Regulation of growth saturation and the development of necrosis in multicell spheroids by the oxygen and glucose supply. Cancer Res 1986; 46: 3504-3512.
Müller-Klieser W, Freyer JP, Sutherland RM. Influence of glucose and oxygen supply conditions on the oxygenation of multicellular spheroids. Br J Cancer 1986; 53: 345-353.
Carlsson J, Acker H. Relations between pH, oxygen partial pressure and growth in cultured cell spheroids. Int J Cancer 1988; 42: 715-720.
Freyer JP, Sutherland RM. Selective dissociation and characterization of cells from different regions of multicell tumor spheroids. Cancer Res 1980; 40: 3956-3965.
Sutherland RM, Sordat B, Bamat J, Gabbert H, Bourrat B, Mueller-Klieser W. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res 1986; 46: 5320-5329.
Bredel-Geissler A, Karbach U, Walenta S, et al. Proliferation-associated oxygen consumption and morphology of tumor cells in monolayer and spheroid culture. J Cell Physiol 1992; 153: 44-52.
Walenta S, Dötsch J, Mueller-Klieser W. ATP concentrations in multicellular tumor spheroids assessed by single photon imaging and quantitative bioluminescence. Eur J Cell Biol 1990; 52: 389-393.
Casciari JJ. The effect of the diffusion and reaction of nutrients and metabolic waste products on the growth and microenvironment of multicellular tumor spheroids. PhD thesis. Rochester, NY (USA): University of Rochester, 1989.
Freyer JP, Schor PL, Jarrett KA, Neeman M, Sillerud LO. Cellular energetics measured by phosphorous nuclear magnetic resonance spectroscopy are not correlated with chronic nutrient deficiency in multicellular tumor spheroids. Cancer Res 1991; 51: 3831-3837.
Bourrat-Floeck B, Groebe K, Mueller-Klieser W. Biological response of multicellular EMT6 spheroids to exogenous lactate. Int J Cancer 1991; 47: 792-799.
Gold R, Schmied M, Giegerich G, et al. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 1994; 71: 219-225.
Boersma AWM, Nooter K, Oostrum RG, Stoter G. Quantification of apoptotic cell with fluorescein isothiocyanate-labeled Annexin V in Chinese hamster ovary cell cultures treated with cisplatin. Cytometry 1996; 24: 123-130.
Mueller-Klieser W. Three-dimensional cell cultures: from molecular mechanisms to clinical applications. Am J Physiol 1997; 273: C1109-C1123.
Clarke PGH. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol 1990; 181: 195-213.
Romero FJ, Zukowski D, Mueller-Klieser W. Glutathione content of V79 cells in two-or three-dimensional culture. Am J Physiol 1997; 272: C1507-C1512.
Raff M. Social controls on cell survival and cell death. Nature 1992; 356: 397-400.
Abren-Martin MT, Vidrich A, Lynch DM, Torgan SR. Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-alpha and ligation of Fas antigen. J Immunol 1995; 115: 4147-4150.
Cotter TG, Lennon SV, Glynn JG, Martin SJ. Cell death via apoptosis and its relationship to growth, development and differentiation of both tumour and normal cells. Anticancer Res 1990; 10: 1153-1159.
O'Rourke JF, Pugh CW, Bartlett SM, Ratcliffe PJ. Identification of hypoxically inducible mRNAs in HeLa cells using differential display PCR. Role of hypoxia-inducible factor-1. Eur J Biochem 1996; 241: 403-410.
Heacock CS, Sutherland RM. Enhanced synthesis of stress proteins caused by hypoxia and relation to altered cell growth and metabolism. Br J Cancer 1990; 62: 217-225.
Scannell G. Leucocyte responses to hypoxia/ischemia conditions. New Horizon 1996; 4: 179-183.
Derevianko A, D'Amico R, Simms H. Polymorphonuclear leucocyte (PMN)-derived inflammatory catokines — regulation by oxygen tension and extracellular matrix. Clin Exp Immunol 1996; 106: 560-567.
Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metabol 1997; 82: 1582-1588.
Kakeji-Y, Maehara-Y, Ikebe-M, Teicher-BA. Dynamics of tumor oxygenation, CD31 staining and transforming growth factor-beta levels after treatment with radiation or cyclophosphamide in the rat 13762 mammary carcinoma. Int J Radiat Oncol Biol Phy 1997; 37: 1115-1123.
Mosser DD, Martin LH. Induced thermotolerance to apoptosis in a human T lymphocyte cell line. J Cell Physiol 1992; 151: 561-570.
Mailhos C, Howard MK, Latchman DS. Heat shock protects neuronal cells from programmed cell death by apoptosis. Neuroscience 1993; 55: 621-627.
Wie YQ, Zhao X, Kariya Y, Teshigawara K, Uchida A. Inhibition of proliferation and induction of apoptosis by abrogation of heat-shock protein (HSP) 70 expression in tumor cells. Cancer Immunol Immunother 1995; 40: 73-78.
Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res 1996; 223: 163-170.
Mehlen P, Schulze-Osthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis. J Biol Chem 1996; 271: 16510-16514.
Yang SH, Nussenzweig A, Li L, et al. Modulation of thermal induction of hsp70 expression by Ku autoantigen or its individual subunits. Mol Cell Biol 1996; 16: 3799-3806.
Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992; 1190: 493-501.
Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colorectal carcinoma cell lines. Cancer Res 1994; 54: 3288-3293.
Hockenberry DM, Zutter M, Hickey W, Nahm M, Korsmeyer S. Bcl-2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci USA 1991; 88: 6961-6965.
Polzar B, Zanotti S, Stephan H, et al. Distribution of deoxyribonuclease I in rat tissues and its correlation to cellular turnover and apoptosis (programmed cell death). Eur J Cell Biol 1994; 64: 200-210.
Hague A, Manning AM, Hanlon KA, Huschtscha LI, Hart D, Paraskeva C. Sodium butyrate induces apoptosis in human colorectal tumor cell lines in a p53-independent pathway: implications for the possible role of dietary fibers in the prevention of largebowel cancer. Int J Cancer 1993; 55: 498-505.
Pinto M, Appay MD, Simson-Assmann P, et al. Enterocytic differentiation of cultured human colon cancer cells by replacement of glucose by galactose in the medium. Biol Cell 1982; 44: 193-196.
Lesufleur T, Barbat A, Dussaulx E, Zweibaum A. Growth adaption to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptove and mucussecreting cells. Cancer Res 1990; 50: 6334-6343.
Lessufleur T, Kornowski A, Luccioni C, et al. Adaptation to 5-fluorouracil of the heterogenous human colon tumor cell line HT-29 results in the selection of cells commited to differentiation. Int J Cancer 1991; 49: 721-730.
Zweibaum A, Pinto M, Chevallier G, et al. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugarfree medium and 1st inhibition by glucose. J Cell Physiol 1985; 122: 21-29.
Lesufleur T, Barbat A, Luccioni C, et al. Dihydropholate reductase gene amplification-associated shift of differentiation in methotrexate-adapted HT-29 cells. J Cell Biol 1991; 115: 1409-1418.
Lessufleur T, Kornowski A, Augeron C, et al. Increased growth adaptability to 5-fluorouracil and methotrexate of HT-29 subpopuplations selected for their commitment to differentiation. Int J Cancer 1991; 49: 731-737.
Augeron C, Laboisse C. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res 1984; 44: 3961-3969.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hauptmann, S., Gebauer-Hartung, P., Leclere, A. et al. Induction of apoptosis in the centre of multicellular tumour spheroids of colorectal adenocarcinomas—involvement of CD95 pathway and differentiation. Apoptosis 3, 267–280 (1998). https://doi.org/10.1023/A:1009613325845
Issue Date:
DOI: https://doi.org/10.1023/A:1009613325845