Abstract
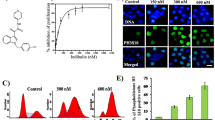
Paclitaxel and docetaxel are potent anti-microtubule and antimitotic agents that induce apoptosis in bone marrow-derived cells and epithelial cells. This study examined apoptosis induced by anti-microtubule agents in the neuroblastoma SK-N-SH cell line with a special focus on tau protein which is one of the main Microtubule-Associated- Proteins (MAPs) in neuronal cells. In time, treatment with 1 μM paclitaxel successively induced formation of bundles, then pseudo-asters concomitantly with mitotic block and phosphorylation of bcl-2 (48 h), then phosphorylation of tau and externalization of phosphatidylserine at the early phase of apoptosis (72 h) and finally DNA fragmentation (96 h). Similar results were obtained with 0.5 μM vinorelbine. Paclitaxel induced a lower increase in tau phosphorylation in differentiated SK-N-SH/RA+ cells which are less sensitive to apoptosis. Moreover, doxorubicin whose mechanism of action is independent of microtubules also induced immunostaining of tau at 72 h treatment. In conclusion, our results on neuroblastoma cells show that overexpression of hyperphosphorylated tau is involved in the apoptotic process induced by anti-microtubule agents and may be extended to others cytostatic drugs. Thus, tau protein may play a role in the cellular events observed in neuroblastoma cells undergoing apoptosis.
Similar content being viewed by others
References
Lee G, Cowan N, Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science 1988; 233: 285-288.
Johnson GV, Jenkins SM. Tau protein in normal and Alzheimer's disease brain. Alzheimers Dis Rev 1996; 1: 38-54.
Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol 1986; 103: 2739-2746.
Mandelkow EM, Mandelkow E. Tau as a marker for Alzheimer's disease. TIBS 1993; 18: 480-483.
Billingsley ML, Kincaid RL. Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J 1997; 323: 577-591.
Avila J, Dominguez J, Diaz-Nido J. Regulation of microtubule dynamics by microtubule-associated protein expression and phosphorylation during neuronal development. Int J Dev Biol 1994; 38: 13-25.
Garcia De Ancos J, Correas I, Avila J. Differences in microtubule binding and self association abilities of bovine brain tau isoforms. J Biol Chem 1993; 268: 7976-7982.
Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron 1991; 6: 487-498.
Mandelkow EM, Biernat J, Drewes G, Gustke N, Trinczek B, Mandelkow E. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol Aging 1995; 16: 355-363.
Mills JC, Lee VMY, Pittman RN. Activation of a PP2A-like phosphatase and dephosphorylation of tau protein characterized onset of the execution phase of apoptosis. J Cell Sci 1998; 111: 625-636.
Tanaka T, Zhong J, Iqbal K, Trenkner E, Grundke-Iqbal I. The regulation of phosphorylation of τ in SY5Y neuroblastoma cells: the role of protein phosphatases. FEBS Lett 1998; 426: 248-254.
Uberti D, Rizzini C, Spano P, Memo M. Characterization of tau proteins in human neuroblastoma SH-SY5Y cell line. Neurosci Lett 1997; 235: 149-153.
Han-Qing X, Johnson GV. Ceramide selectively decreases tau levels in differentiated PC12 cells though modulation of calpain I. J Neurochem 1997; 69: 1020-1030.
Garcia P, Braguer D, Carles G, et al. Comparative effects of taxol and taxotere on two different human carcinoma cell lines. Cancer Chemother Pharmacol 1994; 4: 335-343.
Carles G, Braguer D, Sabeur G, Briand C. The effect of combining anti-tubulin agents on differentiated and undifferentiated human colon cancer cells. Anti-Cancer Drugs 1998; 9: 209-221.
Debal V, Allam N, Morjani H, et al. Characterization of a navelbin-resistant bladder carcinoma cell line cross-resistant to taxoids. Br J Cancer 1994; 70: 1118-1125.
De Ines C, Leynadier D, Barasoain I, et al. Inhibition of microtubules and cell cycle arrest by a new I-deaza-7, 8-dihydropteridine antitumor drug, and by its chiral isomer, NSC 613863. Cancer Res 1994; 54: 75-84.
Garcia P, Braguer D, Carles G, Briand C. Simultaneous combination of microtubule depolymerizing and stabilizing agents acts at low doses. Anti-Cancer Drugs 1995; 6: 533-544.
Van Engeland M, Ramackers FC, Schutte B, Reutelingsperger CP. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996; 24: 131-139.
Remacle-Bonnet MM, Garrouste FL, Pommier GJ. Surface-bound plasmin induces selective proteolysis of Insulin-like-Growth-Factor (IGF)-Binding Protein-4 (IGFBP-4) and promotes autocrine IGF-II bio-availability in human colon-carcinoma cells. Int J Cancer 1997; 72: 835-843.
Lasorella A, Iavarone A, Israel MA. Differentiation of neuroblastoma enhances bcl-2 expression and induces alterations of apoptosis and drug resistance. Cancer Res 1995; 55: 4711-4716.
Babajko S, Binoux M. Modulation by retinoic acid of insulin-like growth factor (IGF) and IGF binding protein expression in human SK-N-SN neuroblastoma cells. Eur J Endocrinol 1996; 134: 474-480.
Cianfarani S, Germani D, Rossi P, Spagnoli A, Mercanti D. Do insulin-like growth factor binding proteins (IGFBPs) modulate the IGF-I growth promoting and differentiating effects in human neuroblastoma cells? Eur J Endocrinol 1996; 135: 716-723.
Hossenlopp P, Segovia B, Lassare C, Roghani M, Bredon M, Binoux M. Evidence of enzymatic degradation of insulin-like growth factor binding proteins in the 150 kD complex during pregnancy. J Clin Endocrinol Metab 1990; 71: 797-805.
Tishler RB, Lamppu DM, Park S, Price BD. Microtubule-active drugs taxol, vinblastine, and nocodazole increase the levels of transcriptionally active p53. Cancer Res 1995; 55: 6021-6025.
Debernardis D, Graniela Sire E, De Feudis P, et al. p53 status does not affect sensitivity of human ovarian cancer cell lines. Cancer Res 1997; 57: 870-874.
Ponzoni M, Bocca P, Chiesa V, et al. Differential effects of N-(4-Hydroxyphenyl) retinamide and retinoic acid on neuroblastoma cells: Apoptosis versus differentiation. Cancer Res 1995; 55: 853-861.
Nuydens R, Heers C, Chadarevian A, et al. Sodium butyrate induces aberrant tau phosphorylation and programmed cell death in human neuroblastoma cells. Brain Res 1995; 688: 86-94.
Vulliet R, Halloran SM, Braun R, Smith A, Lee G. Proline-directed phosphorylation of human tau protein. J Biol Chem 1992; 267: 22570-22574.
Drewes G, Lichten-Kraag B, Doring F, et al. Mitogen-activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J 1992; 11: 2131-2138.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guise, S., Braguer, D., Remacle-Bonnet, M. et al. Tau protein is involved in the apoptotic process induced by anti-microtubule agents on neuroblastoma cells. Apoptosis 4, 47–58 (1999). https://doi.org/10.1023/A:1009682116158
Issue Date:
DOI: https://doi.org/10.1023/A:1009682116158