Abstract
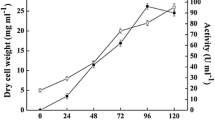
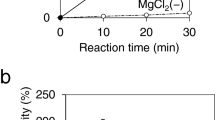
Glucose kinase of Streptomyces coelicolor A3(2) is essential for glucose utilisation and is required for carbon catabolite repression (CCR) exerted through glucose and other carbon sources. The protein belongs to the ROK-family, which comprises bacterial sugar kinases and regulators. To better understand glucose kinase function, we have monitored the cellular activity and demonstrated that the choice of carbon sources did not significantly change the synthesis and activity of the enzyme. The DNA sequence of the Streptomyces lividans glucose kinase gene glkA was determined. The predicted gene product of 317 amino acids was found to be identical to S. coelicolor glucose kinase, suggesting a similar role for this protein in both organisms. A procedure was developed to produce pure histidine-tagged glucose kinase with a yield of approximately 10 mg/l culture. The protein was stable for several weeks and was used to raise polyclonal antibodies. Purified glucose kinase was used to explore protein-protein interaction by surface plasmon resonance. The experiments revealed the existence of a binding activity present in S. coelicolor cell extracts. This indicated that glucose kinase may interact with (an)other factor(s), most likely of protein nature. A possible cross-talk with proteins of the phosphotransferase system, which are involved in carbon catabolite repression in other bacteria, was investigated.
Similar content being viewed by others
References
Angell S, Schwarz E & Bibb MJ (1992) The glucose kinase gene of Streptomyces coelicolor A3(2): its nucleotide sequence, transcriptional analysis and role in glucose repression. Mol. Microbiol. 6: 2833–2844
Angell S, Lewis CG, Buttner MJ & Bibb MJ (1994) Glucose repression in Streptomyces coelicolor A3(2): a likely regulatory role for glucose kinase. Mol. Gen. Genet. 244: 135–143
Baltz RH (1998) Genetic manipulation of antibiotic-producing Streptomyces. Trends Microbiol. 6: 76–83
Bibb M (1996) 1995 Colworth Prize Lecture. The regulation of antibiotic production in Streptomyces coelicolor A3(2). Microbiology 142: 1335–1344
Butler MJ, Deutscher J, Postma PW, Wilson TJ, Galinier A & Bibb MJ (1999) Analysis of a ptsH homologue from Streptomyces coelicolor A3(2). FEMS Microbiol. Lett. 177: 279–288
Chater KF (1993) Genetics of differentiation in Streptomyces. Annu. Rev. Microbiol. 47: 685–713
Chater KF (1998) Taking a genetic scalpel to the Streptomyces colony. Microbiology 144: 1465–1478
Chater KF & Hopwood DA (1983) Streptomyces. In: Sonenshein AL, Hoch JA & Losick R (Eds) Bacillus subtilis and other Gram-positive bacteria (pp 83–99). American Society for Microbiology, Washington DC.
Geourjon C & Deléage G (1995) SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. 11: 681–684
Hindle Z & Smith CP (1994) Substrate induction and catabolite repression of the Streptomyces coelicolor glycerol operon are mediated through the GylR protein. Mol.Microbiol. 12: 737–745
Hodgson DA (1982) Glucose repression of carbon source uptake and metabolism in Streptomyces coelicolor A3(2) and its perturbation in mutants resistant to 2–deoxyglucose. J Gen Microbiol 128: 2417–2430
Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM & Schrempf H (1985) Genetic anipulation of Streptomyces. A laboratory Manual. In: John Innes Foundation, Norwich.
Hopwood DA, Chater KF & Bibb MJ (1995) Genetics of antibiotic production in Streptomyces coelicolor A3(2), a model streptomycete. Biotechnology 28: 65–102
Ingram C, Delic I & Westpheling J (1995) ccrA1: a mutation in Streptomyces coelicolor that affects the control of catabolite repression. J. Bacteriol. 177: 3579–3586
Kwakman JH & Postma PW (1994) Glucose kinase has a regulatory role in carbon catabolite repression in Streptomyces coelicolor. J. Bacteriol. 176: 2694–2698
Mahr K, Hillen W & Titgemeyer F (2000) Carbon catabolite repression in Lactobacillus pentosus: analysis of the ccpA region. Appl. Environ. Microbiol. 66: 277–283
Mattern SG, Brawner ME & Westpheling J (1993) Identification of a complex operator for galP1, the glucose-sensitive, galactosedependent promoter of the Streptomyces galactose operon. J. Bacteriol. 175: 1213–1220
Meyer D, Schneider-Fresenius C, Horlacher R, Peist R & Boos W (1997) Molecular characterization of glucokinase from Escherichia coli K-12. J. Bacteriol. 179: 1298–1306
Parche S, Schmid R & Titgemeyer F (1999) The phosphotransferase system (PTS) of Streptomyces coelicolor: identification and biochemical analysis of a histidine phosphocarrier protein HPr encoded by the gene ptsH. Eur. J. Biochem. 265: 308–317
Pope MK, Green BD & Westpheling J (1996) The bld mutants of Streptomyces coelicolor are defective in the regulation of carbon utilization, morphogenesis, and cell-cell signalling. Mol. Microbiol. 19: 747–756
Postma PW, Lengeler JW & Jacobson GR (1993) Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57: 543–594
Reizer J, Hoischen C, Titgemeyer F, Rivolta C, Rabus R, Stulke J, Karamata D, Saier MH, Jr. & Hillen W (1998) A novel protein kinase that controls carbon catabolite repression in bacteria. Mol. Microbiol. 27: 1157–1169
Roossien FF, Brink J & Robillard GT (1983) A simple procedure for the synthesis of [32P]phosphoenolpyruvate via the pyruvate kinase exchange reaction at equilibrium. Biochim. Biophys. Acta. 760: 185–187
Saier MH, Jr. (1989) Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol. Rev. 53: 109–120
Saito A, Fujii T, Yoneyama T & Miyashita K (1998) glkA is involved in glucose repression of chitinase production in Streptomyces lividans. J. Bacteriol. 180: 2911–2914
Sambrook J, Fritsch EF & Maniatis T (1989) Molecular cloning: a laboratory manual. 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Seok YJ, Sondej M, Badawi P, Lewis MS, Briggs MC, Jaffe H & Peterkofsky A (1997) High affinity binding and allosteric regulation of Escherichia coli glycogen phosphorylase by the histidine phosphocarrier protein, HPr. J. Biol. Chem. 272: 26511–26521
Skarlatos P & Dahl MK (1998) The glucose kinase of Bacillus subtilis. J. Bacteriol. 180: 3222–3226
Späth C, Kraus A & Hillen W (1997) Contribution of glucose kinase to glucose repression of xylose utilization in Bacillus megaterium. J. Bacteriol. 179: 7603–7605
Stülke J & Hillen W(1999) Carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2: 195–201
Titgemeyer F, Reizer J, Reizer A & Saier MH, Jr. (1994a) Evolutionary relationships between sugar kinases and transcriptional repressors in bacteria. Microbiology 140: 2349–2354
Titgemeyer F, Walkenhorst J, Cui X, Reizer J & Saier MH, Jr. (1994b) Proteins of the phosphoenolpyruvate:sugar phosphotransferase system in Streptomyces: possible involvement in the regulation of antibiotic production. Res. Microbiol. 145: 89–92
Titgemeyer F, Walkenhorst J, Reizer J, Stuiver MH, Cui X & Saier MH, Jr. (1995) Identification and characterization of phosphoenolpyruvate: fructose phosphotransferase systems in three Streptomyces species. Microbiology 141: 51–58
van Wezel GP, White J, Young P, Postma PW & Bibb MJ (1997) Substrate induction and glucose repression of maltose utilization by Streptomyces coelicolor A3(2) is controlled by malR, a member of the lacl-galR family of regulatory genes. Mol. Microbiol. 23: 537–549
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahr, K., van Wezel, G.P., Svensson, C. et al. Glucose kinase of Streptomyces coelicolor A3(2): large-scale purification and biochemical analysis. Antonie Van Leeuwenhoek 78, 253–261 (2000). https://doi.org/10.1023/A:1010234916745
Issue Date:
DOI: https://doi.org/10.1023/A:1010234916745