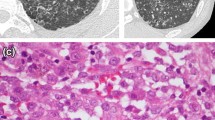
Abstract
The expression of the surface phenotypical profile and the cytokines TNF-α and IL-1β from murine lung macrophages was studied in parenchymal lung tissue and bronchoalveolar fluid of mice, over a 2-week period, following a single intratracheal instillation of silica. The acute inflammatory reaction, confirmed by a significant augmentation of four times the control values of the number of macrophages recovered by lavage from experimental animals, was followed by organized granulomas in the interstitium. The immunohistochemical analysis of lung tissue sections after silica instillation demonstrated the increased alveolar and interstitial tissue expression of all surface antigens and cytokines studied, mainly Mac-1, F4/80 antigens, TNF-α and IL-1β, which were occasionally observed in normal uninjected and saline-treated mice. These findings show that, after silica instillation, the expression of surface phenotypical markers of lung macrophages increased, and this change was concomitantly associated with an increased expression of the cytokines TNF-α and IL-1β. These changes support the conclusion that an influx of the newly recruited and activated macrophage population, with a different phenotype, is induced by treatment during inflammation. The populational changes involve difference in functional activity and enhance TNF-α and IL-1β expression. These cytokines, produced in the silicosis-induced inflammatory process, are associated with the development of fibrosis and may contribute to disease severity. © 1998 Chapman & Hall
Similar content being viewed by others
References
Adamson, I.Y.R. & Bowden, D.H. (1984) Role of polymorphonuclear leukocytes in silica-induced pulmonary fibrosis. Am. J. Pathol. 117, 37-43.
Antonini, J.M., van Dyke, K., Ye, Z., Dimatteo, M. & Reasor M.J. (1994) Introduction of luminol-dependent chemiluminescence as a method to study silica inflammation in the tissue and phagocytic cells of rat lung. Environ. Health Perspect. 102, 37-42.
Austyn, J.M. & Gordon, S. (1981) A monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11, 805-15.
Bilyk, N. & Holt, P.G. (1991) The surface phenotypic characterization of lung macrophages in C3H/HeJ mice. Immunology 74, 645-51.
Bissonnette, E. & Rola-pleszczynski, M. (1989) Pulmonary inflammation and fibrosis in a murine model of asbestosis and silicosis: possible role of tumor necrosis factor. Inflammation 13, 329-39.
Breel, M., van der Ende, M., Sminia, T. & Kraal, G. (1988) Subpopulations of lymphoid and non-lymphoid cells in bronchus-associated lymphoid tissue (BALT) of the mouse. Immunology 63, 657-62.
Bowden, D.H., Hedgecock, C. & Adamson, I.Y.R. (1989) Silica-induced pulmonary fibrosis involves the reaction of particles with interstitial rather than alveolar macrophages. J. Pathol. 158, 73-80.
Calhoun, W. J. & Salisbury, S.M. (1989) Heterogeneity in cell recovery and superoxide production in buyant, density-defined subpopulations of human alveolar macrophages from healthy volunteers and sarcoidosis patients. J. Lab. Clin. Med. 114, 682-90.
Chu, H.W., Wang, J.M., Boutet, M. & Laviolette, M. (1996) Tumor necrosis factor-á and Interleukin-1α expression in a murine model of allergic bronchopulmonary aspergillosis. Lab. Anim. Sci. 46, 42-7.
Carre, P.H., Gossart, S., Rami, J., Cambon, C. & Pipy, B. (1994) Early overexpression of the TNF alpha and Interleukin 1 beta genes by alveolar macrophages in silica-treated rats. Am. J. Resp. Crit. Care Med. 149, 1026A.
Dethloff, L.A., Gilmore, L.B., Gladen, B.C., George, G., Chabra, R.S. & Hook, G.E.R. (1986) Effects of silica on the composition of the pulmonary extracellular lining. Toxicol. Appl. Pharmacol. 84, 66-83.
Dianarello, C.A. (1989) Interleukin-1 and its biologically related cytokines. Adv. Immunol. 44, 153-205.
Driscoll, K.E.R., Lindenschmidt, C., Maurer, J.K., Higgins, J.M., & Ridder, G. (1990a) Pulmonary response to silica or titanium dioxide: inflammatory cells, alveolar macrophage-derived cytokines, and histopathology. Am. J. Respir. Cell. Mol. Biol. 2, 381-90.
Driscoll, K.E., Maurer, J.K., Lindenschmidt, R.C., Romberger, D., Rennard, S. I. & Crosby, L. (1990b) Respiratory tract responses to dust: relationship between dust burden, lung injury, alveolar macrophage fibronectin release, and the development of pulmonary fibrosis. Toxicol. Appl. Pharmacol. 106, 88-101.
Erlandsen, S.L., Hasslen, S.R. & Nelson, R.D. (1993) Detection and spatial distribution of the β2-integrin (Mac-1) and L-Selectin (LECAM-1) adherence receptors on human neutrophils by high-resolution field emission SEM. J. Histochem. Cytochem. 41, 327-33.
Frigeri, L.G. & Liu, F.T. (1992) Surface expression of functional IgE binding protein, an endogen lectin, on mast cells and macrophages. J. Immunol. 148, 861-67.
Gant, V.A. & Hamblin, A.S. (1985) Human bronchoalveolar macrophage heterogeneity demonstrated by histochemistry, surface markers and phagocytosis. Clin. Exp. Immunol. 60, 539-45.
Gordon, S., Crocker, P.R., MORRIS, L., Lee, S.H., Perry, V.H. & Hume, D.A. (1986) Localization and function of tissue macrophages. Ciba Found. Symp. 118, 54-67.
Gossart, S., Cambon, C., Orfila, C., Seguelas, M.H., Lepert, J.C., Rami, J., CarrÉ, P.H. & Pipy, B. (1996) Reactive oxygen intermediates as regulators of TNF-α production in rat lung inflammation induced by silica. J. Immunol. 156, 1540-8.
Hendrzak, J.A. & Morahan, P.S. (1992) Importance of resident peritoneal macrophages in the early inflammatory response to herpes simplex virus type 2 infection. FASEB J. 6, A1714.
Hildemann, S., Hammer, C. & Krombach, F. (1992) Heterogeneity of alveolar macrophages in experimental silicosis. Environ. Health Perpect. 97, 53-57.
Hirsch, S., Austyn, J.M. & Gordon, S. (1981) Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J. Exp. Med. 154, 713-25.
Ho, M.K. & Springer, T.A. (1982) Mac-2, a novel 32000 mw mouse macrophage subpopulation antigen defined by monoclonal antibodies. J. Immunol. 128, 1221-8.
Hocking, W.G. & Golde, W.D. (1979) The pulmonary alveolar macrophage. Part 1. N. Engl. J. Med. 30, 580-7.
Hume, D.A., Perry, V.H. & Gordon, S. (1984) The mononuclear phagocyte system of the mouse defined by immunohistological localisations of antigen F4/80: Macrophages associated with epithelia. Anat. Rec. 210, 503-12.
Johard, U., Eklund, A., Hed, J. & Lundahl, J. (1993) Terpenes enhance metabolic activity and alter expression of adhesion molecules (Mac-1 and L-selectin) on human granulocytes. Inflammation 4, 499-509.
Kasper, M. & Hughes, R.C. (1996) Immunocytochemical evidence for a modulation of galectin 3 (Mac-2), a carbohydrate binding protein, in pulmonary fibrosis. J. Pathol. 179, 309-16.
Kraal, G., Rep, M. & Janse, M. (1987) Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. An immunohistochemical study. Scand. J. Immunol. 26, 653-61.
Leenen, P.J.M., De Bruijn, M.F.T.R., Voerman, J.S.A., Campbell, P.A. & van Ewijk, W. (1994) Markers of mouse macrophage development detected by monoclonal antibodies. J. Immunol. Meth. 174, 5-19.
Lotz, M.M., Andrews, C.W., Korzelius, C.A. Lee, E.C., Steele, G.D., Clarke, A. & Mercurio, A.M. (1993) Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc. Natl. Acad. Sci. USA 90, 3466-70.
Menson, E.N. & Wilson, R.A. (1990) Lung-phase immunity to Schistosoma mansoni: definition of alveolar macrophage phenotypes after vaccination and challenge of mice. Parasite Immunol. 12, 353-66.
Miles, P., Bowman, R.L., Jones, W.G., Berry, D.S. & Vallyathan, V. (1994) Changes in alveolar lavage materials and lung microsomal xenobiotic metabolism following exposures to HCl-washed crystalline silica. Toxicol. Appl. Pharmacol. 129, 235-42.
Miller, K., Hudspith, B.N. & Meredith, C. (1992) Secretory and accessory cell functions of the alveolar macrophage. Environ. Health Perspect. 97, 85-9.
Mori, H., Tanaka, H., Kawada, K., Nagai, H. & Koda, A. (1995) Suppressive effects of tranilast on pulmonary fibrosis and activation of alveolar macrophages in mice treated with bleomycin: role of alveolar macrophages in the fibrosis. Jpn. J. Pharmacol. 67, 279-89.
Nibbering, P.H., Leijh, P.C.J. & van Furth, R. (1987a) Quantitative immunocytochemical characterization of mononuclear phagocytes. II. Monocytes and tissue macrophages. Immunology 62, 171-6.
Nibbering, P.H., Leijh, P.C.J. & van Furth, R. (1987b) Quantitative immunocytochemical characterization of mononuclear phagocytes. I. Monoblasts, promonocytes, monocytes and peritoneal and alveolar macrophages. Cell. Immunol. 105, 374-85.
Nibbering, P.H., Van De Heide G.A. & Van Furth, R. (1989) Macrophages in bronchoalveolar lavagefluid do not represent macrophages in granulomas of the lungs of BCG-infected mice. Agents Actions 26, 132-3.
Oghiso, Y. & Kubota, Y. (1987) Interleukin 1 production and accessory cell function of rat alveolar macrophages exposed to mineral dust particles. Microbiol. Immunol. 31, 275-87.
Piguet, P.F., Collart, M.A., Grau, G.E., Sappino, A.P. & Vassalli, P. (1990) Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature 344, 245-7.
Plasman, N. & Vray, B. (1994) Immunophenotyping of murine peritoneal macrophages and lymphocytes by flow cytometry. J. Immunol. Methods 174, 123-31.
Reiser, K.M., Westerberg, T.W., Haschek, W.M. & Last, J.A. (1983) Experimental silicosis: II. Long-term effects of intratracheally instilled quartz on collagen metabolism and morphologic characteristics of rat lungs. Am. J. Pathol. 110, 30-41.
Rutherford, M. S., Witsel, A. & Shook, L.B. (1993) Mechanisms generating functionally heterogeneous macrophages: Chaos revisited. J. Leukocyte Biol. 53, 602-13.
Sarih, M., Souvannavong, V., Brown, S.C. & Adam, A. (1993) Silica induces apoptosis in macrophages and the release of interleukin-1á and interleukin-1β. J. Leukocyte Biol. 54, 407-13.
Schollmeier, K. (1990) Immunological and pathophysiologic role of tumor necrosis factor. Am. J. Respir. Cell. Mol. Biol. 3, 13-20.
Shellito, J. & Kaltreider, H.B. (1985) Heterogeneity of immunologic function among subfractions of normal rat alveolar macrophages. II. Activation as determinant of functional activity. Am. Rev. Respir. Dis. 131, 678-83.
Springer, T., Galfre, G., Secher, D.S. & Milstein, C. (1979) Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur. J. Immunol. 9, 301-6.
Walker, E.B., Akporiaye, E.T., Warner, N.L. & Stewart, C.C. (1985) Characterization of subsets of bone marrow-derived macrophages by flow cytometry analysis. J. Leukocyte Biol. 37, 121-136.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Orfila, C., Lepert, J.C., Gossart, S. et al. Immunocytochemical Characterization of Lung Macrophage Surface Phenotypes and Expression of Cytokines in Acute Experimental Silicosis in Mice. Histochem J 30, 857–867 (1998). https://doi.org/10.1023/A:1003485312164
Issue Date:
DOI: https://doi.org/10.1023/A:1003485312164