Abstract
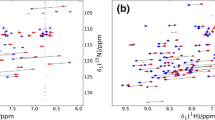
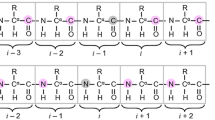
Many protein–protein interactions involve amino acid sequences containing proline-rich motifs and even poly-proline stretches. The lack of amide protons in such regions complicates assignment, since 1HN-based triple-resonance assignment strategies cannot be employed. Two such systems that we are currently studying include an SH2 domain from the protein Crk with a region containing 9 prolines in a 14 amino acid sequence, as well as a WW domain that interacts with a proline-rich target. A modified version of the HACAN pulse scheme, originally described by Bax and co-workers [Wang et al. (1995) J. Biomol. NMR, 5, 376–382], and an experiment which correlates the intra-residue 1Hα, 13Cα/13Cβ chemical shifts with the 15N shift of the subsequent residue are presented and applied to the two systems listed above, allowing sequential assignment of the molecules.
Similar content being viewed by others
References
Alexandropoulos, K., Cheng, G. and Baltimore, D. (1995) Proc. Natl. Acad. Sci. USA, 92, 3110–3114.
Bedford, M.T., Chan, D.C. and Leder, P. (1997) EMBO J., 16, 2376–2383.
Bedford, M.T., Reed, R. and Leder, P. (1998) Proc. Natl. Acad. Sci. USA, 95, 10602–10607.
Bottomley, M.J., Macias, M.J., Liu, Z. and Sattler, M. (1999) J. Biomol. NMR, 13, 381–385.
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR, 6, 277–293.
Ermekova, K.S., Zambrano, N., Linn, H., Minopoli, G., Gertler, F., Russo, T. and Sudol, M. (1997) J. Biol. Chem., 272, 32869–32877.
Feller, S.M., Knudsen, B. and Hanafusa, H. (1994) EMBO J., 13, 2341–2351.
Freund, C., Dötsch, V., Nishizawa, K., Ellis, L., Reinherz, E.L. and Wagner, G. (1999) Nat. Struct. Biol., 6, 656–660.
Garrett, D.S., Powers, R., Gronenborn, A.M. and Clore, G.M. (1991) J. Magn. Reson., 95, 214–220.
Geen, H. and Freeman, R. (1991) J. Magn. Reson., 93, 93–141.
Gertler, F.B., Niebuhr, K., Reinhard, M., Wehland, J. and Soriano, P. (1996) Cell, 87, 227–239.
Johnson, B.A. and Blevins, R.A. (1994) J. Biomol. NMR,4, 603–614.
Kanelis, V., Farrow, N.A., Kay, L.E., Rotin, D. and Forman-Kay, J.D. (1998) Biochem. Cell. Biol., 76, 341–350.
Kay, L.E. (1993) J. Am. Chem. Soc., 115, 2055–2057.
Kay, L.E., Ikura, M., Tschudin, R. and Bax, A. (1990) J. Magn. Reson., 89, 496–514.
Kay, L.E., Keifer, P. and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663–10665.
Marion, D., Ikura, M., Tschudin, R. and Bax, A. (1989) J. Magn. Reson., 85, 393–399.
McCoy, M.A. and Mueller, L. (1992a) J. Am. Chem. Soc., 114, 2108–2112.
McCoy, M.A. and Mueller, L. (1992b) J. Magn. Reson., 98, 674–679.
Niebuhr, K., Ebel, F., Frank, R., Reinhard, M., Domann, E., Carl, U.D., Walter, U., Gertler, F.B., Wehlend, J. and Chakraborty, T. (1997) EMBO J., 16, 5433–5444.
Olejniczak, E.T. and Fesik, S.W. (1994) J. Am. Chem. Soc., 116, 2215–2216.
Palmer, A.G., Cavanagh, J., Wright, P.E. and Rance, M. (1991) J. Magn. Reson., 93, 151–170.
Pawson, T. (1995) Nature, 373, 573–580.
Reinhard, M., Giehl, K., Abel, K., Haffner, C., Jarchau, T., Hoppe, V., Jockusch, B.M. and Walter, U. (1995) EMBO J., 14, 1583–1589.
Rosen, M.K., Yamazaki, T., Gish, G.D., Kay, C.M., Pawson, T. and Kay, L.E. (1995) Nature, 374, 477–479.
Rozakis-Adcock, M., Fernley, R., Wade, J., Pawson, T. and Bowtell, D. (1993) Nature, 363, 83–85.
Santoro, J. and King, G.C. (1992) J. Magn. Reson., 97, 202–207.
Schleucher, J., Sattler, M. and Griesinger, C. (1993) Angew. Chem. Int. Ed. Engl., 32, 1489–1491.
Shaka, A.J., Keeler, J., Frenkiel, T. and Freeman, R. (1983) J. Magn. Reson., 52, 335–338.
Staub, O., Dho, S., Henry, P., Correa, J., Ishikawa, T., McGlade, J. and Rotin, D. (1996) EMBO J., 15, 2371–2380.
Staub, O. and Rotin, D. (1996) Structure, 4, 495–499.
Vuister, G.W. and Bax, A. (1992) J. Magn. Reson., 98, 428–435.
Wang, A.C., Grzesiek, S., Tschudin, R., Lodi, P. and Bax, A. (1995) J. Biomol. NMR, 5, 376–382.
Wishart, D.S., Bigam, C.G., Holm, A., Hodges, R.S. and Sykes, B.D. (1995) J. Biomol. NMR, 5, 67–81.
Yu, H., Chen, J.K., Feng, S., Dalgarno, D.C., Brauer, A.W. and Schreiber, S.L. (1994) Cell, 76, 933–945.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kanelis, V., Donaldson, L., Muhandiram, D. et al. Sequential assignment of proline-rich regions in proteins: Application to modular binding domain complexes. J Biomol NMR 16, 253–259 (2000). https://doi.org/10.1023/A:1008355012528
Issue Date:
DOI: https://doi.org/10.1023/A:1008355012528