Summary
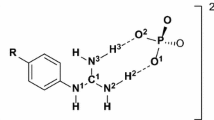
A purine derivative with an acyclic sugar analog, 3,9-dihydro-3-[(2-hydroxyethoxy)methyl]-6-ethyl-9-oxo-5H-imidazo[1,2-a]purine, was studied in the free state and in complex with herpes simplex virus thymidine kinase (HSV1 TK). Transferred NOE experiments, combined with a full relaxation matrix analysis of the substrate's spin system, resulted in a set of distance constraints for all proton pairs. These constraints were used in structure determination procedures based on simulated annealing and molecular dynamics simulations to obtain a family of structures compatible with the experimental NMR data. The results indicate that, although in both states the chains have the syn orientation with respect to the aromatic rings, in the free state the substrate's acyclic moiety is relatively disordered, while in the bound state only one specific conformation is preferred. Fluctuations can only be seen in the case of the terminal hydroxyl group, for which no NOE was recorded and hence no constraints were available.
Similar content being viewed by others
References
Balaram, P., Bothner-By, A.A. and Dadok, J. (1972) J. Am. Chem. Soc., 94, 4015–4016.
Biosym Technologies, Inc., San Diego, CA, U.S.A., NMRchitect User Guide, v. 2.3, 1993.
Black, M.E. and Loeb, L.A. (1993) Biochemistry, 32, 11618–11626.
Boryski, J., Golankiewicz, B. and DeClercq, E. (1991) J. Med. Chem., 34, 2380–2383.
Campbell, A.P. and Sykes, B.D. (1991) J. Magn. Reson., 93, 77–92.
Clore, G.M. and Gronenborn, A.M. (1982) J. Magn. Reson., 48, 402–417.
Czaplicki, J., Michael, M., Folkers, G. and Milon, A. (1995) J. Chim. Phys., 92, 1773–1776.
Edmondson, S.P. (1992) J. Magn. Reson., 98, 283–298.
Eriksson, S., Kierdaszuk, B., Munch-Petersen, B., Öberg, B. and Johansson, N.G. (1991) Biochem. Biophys. Res. Commun., 176, 586–592.
Fetzer, J. and Folkers, G. (1992) Pharm. Pharmacol. Lett., 2, 112–114.
Fetzer, J., Folkers, G., Müller, I. and Keil, G.M. (1993) Virus Genes, 7, 205–209.
Fetzer, J., Michael, M., Bohner, T., Hofbauer, R. and Folkers, G. (1994) Protein Expr. Purif., 5, 432–441.
Fitt, P.S., Peterkin, P.I. and Grey, V.L. (1976) J. Chromatogr., 124, 137–140.
Folkers, G., Trumpp-Kallmeyer, S., Gutbrod, O., Krickl, S., Fetzer, J. and Keil, G.M. (1991) J. Comput.-Aided Mol. Design, 5, 385–404.
Furlong, N.B. (1963) Anal. Biochem., 5, 515–522.
Fyfe, J.A., Keller, P.M., Furman, P.A., Miller, R.L. and Elion, G.B. (1978) J. Biol. Chem., 253, 8721–8727.
Huber, B.E., Richards, C.A. and Krenitsky, T.A. (1991) Proc. Natl. Acad. Sci. USA, 88, 8039–8043.
James, T.L. (1994) Methods Enzymol., 239, 416–439.
Keller, P.M. and Elion, G.B. (1981) Biochem. Pharmacol., 30, 3071–3077.
Lian, L.Y., Barsukov, I.L., Sutcliffe, M.J., Sze, K.H. and Roberts, G.C.K. (1994) Methods Enzymol., 239, 657–699.
Liu, H., Thomas, P.D. and James, T.L. (1992) J. Magn. Reson., 98, 163–175.
London, R.E., Perlman, M.E. and Davis, D.G. (1992) J. Magn. Reson., 97, 79–98.
Michael, M., Fetzer, J. and Folkers, G. (1994) Eur. J. Biochem., 226, 219–226.
Miller, W.H. and Miller, R.L. (1980) J. Biol. Chem. 255, 7204–7207.
Munch-Petersen, B., Cloos, L., Tyrsted, G. and Eriksson, S. (1991) J. Biol. Chem., 266, 9032–9038.
Neuhaus, D. and Williamson, M. (1989) The Nuclear Overhauser Effect in Structural and Conformational Analysis, VCH Publishers, New York, NY, U.S.A.
Nilges, M., Clore, G.M. and Gronenborn, A.M. (1988) FEBS Lett., 239, 129–136.
Ohno, T., Gordon, D., San, H., Pompili, V.J., Imperiale, M.J., Nabel, G.J. and Nabel, E.G. (1994) Science, 265, 781–784.
Reardon, J.E. and Spector, T. (1989) J. Biol. Chem., 264, 7405–7411.
Tropp, J. (1980) J. Chem. Phys., 72, 6035–6043.
Zangwill, W.I. (1969) Nonlinear Programming: A Unified Approach, Prentice-Hall, Englewood Cliffs, NJ, U.S.A.
Zimmermann, N., Beck-Sickinger, A.G., Folkers, G., Krickl, S., Müller, I. and Jung, G. (1991) Eur. J. Biochem. 200, 519–528.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Czaplicki, J., Bohner, T., Habermann, AK. et al. A transferred NOE study of a tricyclic analog of acyclovir bound to thymidine kinase. J Biomol NMR 8, 261–272 (1996). https://doi.org/10.1007/BF00410325
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00410325