Abstract
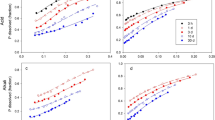
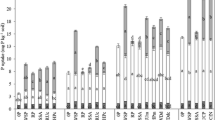
The influences of calcium chloride, calcium sulphate, monocalcium phosphate, monosodium phosphate, calcium carbonate, sodium carbonate and iron and aluminium sulphates on the solubilities of two phosphate rocks (both ground and unground) in 2% citric and 2% formic acids have been examined. All of these potential ‘impurity’ elements can affect the solubility of the phosphate rock through a variety of mechanisms, including common ion effects, complexation, acid-base reactions and reduction. 2% formic acid solubilities were slightly more influenced by these effects than 2% citric acid figures, militating against the use of this extractant for assessing mixed component products at present being manufactured in New Zealand.
Solid to solvent and P to solvent ratios were also found to affect the solubilities of raw phosphate rocks and a correction for the differences in total P content of the phosphate rocks to produce a consistent P to solvent ratio in solubility tests is suggested. This study has indicated that alternative methods for assessing phosphate rocks in admixture with other components should be examined in future.
Similar content being viewed by others
References
Braithwaite AC (1987) The Use of Chemical Solubility Tests in Comparing Phosphate Fertilisers. Fert Res 12: 185–192
Braithwaite AC and Rogers DA (1985) The Reliability and Appropriateness of Chemical Solubility Tests for Phosphate Fertilisers, NZFMRA 9th Res. Symp Proc 3–15
Mackay AD, Syers JK and Gregg PEH (1984) Ability of Chemical Extraction Procedures to Predict the Agronomic Effectiveness of Phosphate Rock Materials. NZJ Agric Res 27: 219–230
Quin BF (1982) The Use of Citric Acid Soluble and Water Soluble Phosphate to Assess the Agronomic Value of Fertilisers, NZFMRA 7th Res Symp Proc 40–54
Stephen RC (1982) The Relationship Between Phosphorus Solubility of Single Superphosphate in Different Extractants and the Dry Matter Production of Ryegrass in Pots, NZFMRA 7th Res Symp Proc 24–31
Syers JK, Mackay AD, Brown MW and Currie LD (1986) Chemical and Physical Characteristics of Phosphate Rock Materials of Varying Reactivity. J Sci Food Agric 37: 1057–1064
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Braithwaite, A.C., Eaton, A.C. & Groom, P.S. Some factors associated with the use of the extractants 2% citric acid and 2% formic acid as estimators of available phosphorus in fertiliser products. Fertilizer Research 19, 175–181 (1989). https://doi.org/10.1007/BF01054459
Issue Date:
DOI: https://doi.org/10.1007/BF01054459