Abstract
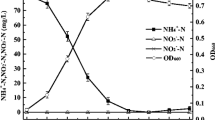
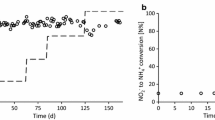
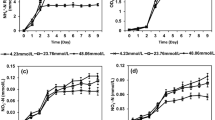
An in situ method for measuring nitrate reductase (NR) activity in Dunaliella viridis was optimized in terms of incubation time, concentration of KNO3, permeabilisers (1-propanol and toluene), pH, salinity, and reducing power (glucose and NADH). NR activity was measured by following nitrite production and was best assayed with 50 mM KNO3, 1.2 mM NADH, 5% 1-propanol (v/v), at pH 8.5. The estimated half-saturation constant (Ks) for KNO3 was 5 mM. Glucose had no effect as external reducing power source, and NADH concentrations >1.2 mM inhibited NR activity. Nitrite production was linear up to 20 min; longer incubation did not lead to higher nitrate reduction. The use of the optimized assay predicted the rate of NO −3 removal from the external medium by D. viridis with high degree of precision.
Similar content being viewed by others
References
Aryan AP, Batt RG, Wallace W (1983) Reversible inactivation of nitrate reductase by NADH and the occurrence of partially inactive enzyme in the Wheat Leaf. Plant Physiol. 71: 582–587.
Berges JA, Harrison PJ (1995a) Nitrate reductase activity quantitatively predicts the rate of nitrate incorporation under steady state light limitation: A revised assay and characterization of the enzyme in three species of marine phytoplankton. Limnol. Oceanogr. 40: 82–93.
Berges JA, Harrison PJ (1995b) Relationships between nitrate reductase activity and nitrate incorporation under steady-state light or nitrate limitation in the marine diatom Thalassiosira pseudonana (Bacillarophyceae). J. Phycol. 31: 85–95.
Blasco D, MacIsaac JJ, Packard TT, Dugdale RC (1984) Relationship between nitrate reductase and nitrate uptake in phytoplankton in the Peru upwelling region. Limnol. Oceanogr. 29: 275–286.
Blasco D, Packard TT (1974) Nitrate reductase measurements in upwelling regions: 1. Significance of the distribution off Baja California. Tethys 6: 239–246.
Borowitzka MA, Borowitzka LJ (1988) Dunaliella. In Borowitzka MA & Borowitzka LJ (eds), Microalgal Biotechnology. Cambridge University Press, 27–58.
Brinkhuis H, Renzhi L, Chaoyuan W, Xun-Sen J (1989) Nitrite uptake transients and consequences for in vivo algal nitrate reductase assays. J. Phycol. 25: 539–545.
Canvin DT, Woo KC (1979) The regulation of nitrate reduction in spinach leaves. Can. J. Bot. 57: 1155–1160.
Corzo A, Niell FX (1991) Determination of nitrate reductase activity in Ulva rigida C. Agardh by the in situ method. J. exp. mar. Biol. Ecol. 146: 181–191.
Corzo A, Plasa R, Ullrich WR (1991) Extracellular ferricyanide reduction and nitrate reductase activity in the green alga Monoraphidium braunii. Plant Sci. 75: 221–228.
Davison IR, Stewart WDP (1984) Studies on nitrate reductase activity in Laminaria digitata (Huds.) Lamour. II. The role of nitrate availability in the regulation of the enzyme activity. J. exp. mar. Biol. Ecol. 79: 65–78.
Eppley RW, Coatsworth JL, Solorzano L (1969) Studies of nitrate reductase in marine phytoplankton. Limnol. Oceanogr. 14: 194–205.
Eppley RW, Packard TT, McIsaac JJ (1970) Nitrate reductase in Peru current phytoplankton. Mar. Biol. 6: 135–139.
Hernandez I, Corzo A, Gordillo FJL, Robles MD, Saez E, Fernandez JA, Niell FX (1993) Seasonal cycle of the gametophytic form of Porphyra umbilicalis: nitrogen and carbon. Mar. Ecol. Prog. Ser. 99: 301–311.
Hochman A, Nissany A, Wynne D, Kaplan B, Berman T (1986) Nitrate reductase: an improved assay method for phytoplankton. J. Plankton Res. 8: 385–392.
Hurd CL, Berges JA, Osborne J, Harrison PJ (1995) An in vitro nitrate reductase assay for marine macroalgae: optimization and characterization of the enzyme for Fucus gardneri (Phaeophyta). J. Phycol. 31: 835–843.
Javor B (1989) Hypersaline Environments. Brock/Springer Series in Contemporary Biosciences, 328 pp.
Jiménez C, Niell FX (1991) Growth of Dunaliella viridis Teodoresco: effect of salinity, temperature and nitrogen concentration. J. appl. Phycol. 3: 319–327.
Johnson MK, Johnson RD, Macelroy RD, Speer HL, Bruff BS (1968) Effects of salts on the halophilic alga Dunaliella viridis. J. Bact. 95: 1461–1468.
Katz A, Avron M (1985) Determination of intracellular osmotic volume and sodium concnetration in Dunaliella. Plant Physiol. 78: 817–820.
Lawrence JM, Herrick HE (1982) Media for in vivo nitrate reductase assay of plant tissue. Plant Sci. Lett. 24: 273–275.
Lorimer GH, Gewitz HS, Völker W, Solomonson LP, Vennesland B (1974) The presence of bound cyanide in the naturally inactivated form of nitrate reductase of Chlorella vulgaris. J. biol. Chem. 249: 6074–6079.
Mann AF, Hucklesby DP, Hewitt EJ (1979) Effect of aerobic and anaerobic conditions on the in vivo nitrate reductase assay in spinach leaves. Planta 146: 83–89.
Mauriño SG, Echevarria C, Mejias JA, Vargas MA, Maldonado JM (1986) Properties of the in vivo nitrate reductase activity assay in maize, soybean and spinach leaves. J. Plant Physiol. 24: 123–130.
Mauriño SG, Echevarria C, Vargas MA, Maldonado JM (1985) Freezing and thawing on in situ nitrate reductase activity in spinach leaves. J. Plant Physiol. 120: 409–417.
Moreno CG, Aparicio PJ, Palacián E, Losada M (1972) Interconversion of the active and inactive forms of Chlorella nitrate reductase. FEBS Lett. 26: 11–14.
Packard TT, Dugdale RC, Goering JJ, Barber JJ (1978) Nitrate reductase activity in the subsurface waters of the Peru current. J. Mar. Res. 36: 59–76.
Pistorius EK, Gewitz HS, Voss H, Vennesland B (1976) Reversible inactivation of nitrate reductase in Chlorella vulgaris in vivo. Planta 128: 73–80.
Rhodes D, Stewart GR (1974) A procedure for the in vivo determination of enzyme activity in higher plant tissue. Planta 118: 133–144.
Scheidler L, Ninneman H (1986) Nitrate reductase activity: phenazine methosulfate-ferricyanide stop reagent replaces postassay treatment. Analyt. Biochem. 154: 29–33.
Scholl RL, Harper JE, Hageman RH (1974) Improvements of the nitrite color development in assays of nitrate reductase by phenazinemethosulfate and zinc acetate. Plant Physiol. 124: 123–130.
Shinn JA (1941) Ind. Eng. Chem. (annual edition), 13: 33. In Strickland JDH & Parsons TR (eds) A practical Approach Handbook of Seawater Analysis. Fish. Res. Bd Can. Bull. 167.
Snell FD, Snell CT (1949) Colorimetric Methods of Analysis. 3rd Edition, Van Nostrand, Princeton, New Jersey. Vol. 2, 804 pp.
Sym GJ (1984) Optimisation of the in vivo assay conditions for nitrate reductase in barley (Hordeum vulgare L. cv. Igri). J. Sci. Food Agric. 35: 725–730.
Syrett PJ, (1981) Nitrogen metabolism of microalgae. In Platt T (ed.) Physiological Bases of Phytoplankton Ecology. Bull. No. 210182-210. Canadian Government Publishing Center.
Wood ED, Armstrong FAJ, Richards F (1967) Determination of nitrate in seawater by cadmium-copper reduction to nitrite. J. mar. biol. ASS. UK. 47: 23–31.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Javier, F., Gordillo, L., Jiménez, C. et al. Optimized nitrate reductase assay predicts the rate of nitrate utilization in the halotolerant microalga Dunaliella viridis. Journal of Applied Phycology 9, 99–106 (1997). https://doi.org/10.1023/A:1007978512458
Issue Date:
DOI: https://doi.org/10.1023/A:1007978512458