Abstract
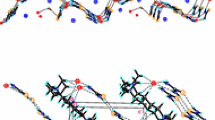
Trimesic acid (benzene-1, 3,5-tri-carboxylic acid; TMA) can in principle form two-dimensional hydrogen-bonded hexagonal networks in which central holes of the network have net diameters of 14 Å. Although such holes would be expected to be natural locations for guest molecules, non-catenated single networks have not been found in any of the crystals containing TMA studied in the last sixteen years. Instead, anhydrous α-TMA, TMA pentaiodide (TMA.I5) and (so-called) γ-TMA have mutually triply-catenated structures in which triplets of networks are interlaced [3,4,5], while the hydrated complexes are based on non-catenated nets of composition TMA.H2O [6]. We have now found conditions under which single networks are preserved without catenation, the cavities being occupied by guests such as n-tetradecane, n-heptanol, n-octanol, n-decanol, octene, cyclooctane and isooctane. The structures of 2TMA. n-tetradecane and 2TMA. isooctane have been solved and refined to R=13.0% and R=11.3%, respectively, disorder of the guest molecules having prevented further refinement of the room-temperature data. Determination of the crystal structures of the other complexes, which are isostructural with 2TMA. n-tetradecane, is now in progress. We are also investigating other potential guests.
Similar content being viewed by others
References
K. Takemoto and N. Sonoda, ‘Inclusion Compounds of Urea, Thiourea and Selenourea’ inInclusion Compounds: Structural Aspects of Inclusion Compounds Formed by Organic Host Lattices. Vol. 2, pp. 47–67 (1984), edited by J. L. Atwood, J. E. D. Davies and D. D. MacNicol, Academic Press, London etc.
M. Farina, ‘Inclusion Compounds of Urea, Thiourea and Selenourea’ inInclusion Compounds: Structural Aspects of Inclusion Compounds Formed by Organic Host Lattices. Vol. 2, pp. 69–95. (1984), edited by J. L. Atwood, J. E. D. Davies and D. D. MacNicol, Academic Press, London
D. J. Duchamp and R. E. Marsh,Acta Cryst., B25, 5 (1969).
F. H. Herbstein, M. Kapon and G. M. Reisner,Proc. Roy. Soc. Lond., A376, 301 (1981).
F. H. Herbstein, M. Kapon and G. M. Reisner,Acta Cryst., B41, 348 (1985).
F. H. Herbstein and R. E. Marsh,Acta Cryst., B 33, 2358 (1977).
P. Main, M. M. Woolfson, L. Lessinger, G. Germain and J. P. Declercq, “MULTAN 77. A system of computer programmes for the automatic solution of crystal structures for X-ray diffraction data”. Universities of York, England and Louvain, Belgium (1977).
G. M. Sheldrick: “SHELX 77. A programme for crystal structure determination”. University of Cambridge, England (1977).
Johnson, C.K. ORTEP. Report ORNL-3794 Oak Ridge National Laboratory, Tennessee (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Herbstein, F.H., Kapon, M. & Reisner, G.M. Catenated and non-catenated inclusion complexes of trimesic acid. Journal of Inclusion Phenomena 5, 211–214 (1987). https://doi.org/10.1007/BF00655650
Issue Date:
DOI: https://doi.org/10.1007/BF00655650