Summary
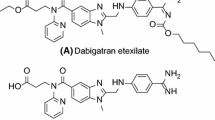
Antagonists of the platelet fibrinogen receptor (GP IIb/IIIa receptor) are expected to be a new promising class of antithrombotic agents. The binding of fibrinogen to the fibrinogen receptor depends on an Arg-Gly-Asp-Ser (RGDS) tetrapeptide recognition motif. Structural modifications of the RGDS lead have led to the discovery of a non-peptide RGD mimetic GP IIb/IIIa antagonist20 (S 1197). Compound20 inhibits dose-dependently and reversibly human platelet aggregation. Modeling studies based on structure-activity data revealed the following structural features of the drug as important for receptor binding: the amidino group, the carboxylate group, hydrophobic substitutions at the carboxyl-terminus and at the side chain carrying the positive charge, the carboxyl-terminal NH group of the β-amino acid as a hydrogen bond donor and one oxygen atom of the hydantoin as a hydrogen bond acceptor. The ethyl ester prodrug of20 (S 5740) is an orally active antithrombotic agent which has the potential to be used to treat and prevent thrombotic diseases in humans.
Similar content being viewed by others
References
Fitzgerald, D.J., Roy, L., Catella, F. and Fitzgerald, G.A., New Engl. J. Med., 315 (1986) 983.
Fuster, V., Steele, P.M. and Chesebro, J.H., J. Am. Coll. Cardiol., 5 (1985) 175B.
Harrison, M.J.G., Circulation, 81 (Suppl. I) (1990) I-20.
Davies, M.J. and Thomas, A.C., Br. Heart J. 53 (1985) 363.
Phillips, D.R., Charo, I.F., Parise, L.V. and Fitzgerald, L.A., Blood, 71 (1988) 831.
Hawiger, J., Hum. Pathol., 18 (1987), 111.
Cook, N.S., Kottirsch, G. and Zerwes, H.-G., Drugs Future, 19 (1994) 135.
Weller, T., Alig, L., Hürzeler Müller, M., Kouns, W.C., and Steiner, B., Drugs Future, 19 (1994) 461.
Austel, V., Himmelsbach, F. and Müller, T., Drugs Future, 19 (1994) 757.
Lefkovits, J., Plow, E.F. and Topol, E.J., New Engl. J. Med., 332 (1995) 1553.
The EPIC investigators, New Engl. J. Med., 330 (1994) 956.
Ruoslahti, E., J. Clin. Invest., 87 (1991), 1.
Just, M., Hropot, M., Jablonka, B., König, W., and Stilz, H.U., Thromb. Haemost., 73 (1995) 1444.
Jablonka, B., Just, M., König, W. and Stilz, H.U., Naunyn-Schmied. Arch. Pharmacol., 353s (1995) R29 (116).
Stilz, H.U., Jablonka, B., Just, M., Knolle, J., Paulus, E.F. and Zoller, G., J. Med. Chem., 39 (1996) 2118.
Stilz, H.U., Beck, G., Just, M. and Jablonka, B., Bull. Soc. Chim. Belg., 105 (1996) 711.
Brooks, B., Bruccoleri, R., Olafson, B., States, D., Swaminathan, S., and Karplus, M., J. Comput. Chem., 4 (1983) 187.
Catalyst, HipHop, v.3.1, Molecular Simulations, San Diego, CA, U.S.A., 1996.
SYBYL, Molecular Modeling Software, v. 6.2, Tripos Inc., St. Louis, MO, U.S.A., 1995.
Cramer III, R.D., Patterson, D.E. and Bunce, J.D., J. Am. Chem. Soc., 110 (1988) 5959.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stilz, H.U., Guba, W., Jablonka, B. et al. From a peptide lead to an orally active peptidomimetic fibrinogen receptor antagonist. Lett Pept Sci 5, 215–221 (1998). https://doi.org/10.1007/BF02443472
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02443472