Summary
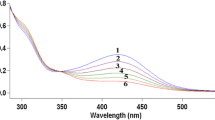
The kinetics of reduction of [MnIII(cydta)]− (where H4cydta=trans-cyclohexane-1,2-diamine-N N N' N'-tetraacetic acid) by some thiourea reductants have been studied in aqueous solution by stopped-flow techniques in the pH ranges 2.5–4.5 and 9.2–10.2. An initial increase in absorbance followed by a steady decrease indicated the formation of a precursor complex prior to the electron transfer step. The reactions are first order in both oxidant and reductant. The observed increase in rate in going from low to high pH is attributed to the difference in reactivities of the aqua and hydroxo species of the MnIII complex; the higher reactivity of the latter is consistent with the formation of a ligand-bridged activated species prior to electron transfer. The reactivity order for the thiourea derivatives follows the order of their reported substituent effects.
Similar content being viewed by others
References
G. M. Cheniae and I. F. Martin,Biochim. Biophys. Acta,197, 219 (1970).
H. Metzner (Ed.),Photosynthetic Oxygen Evolution, Academic Press, New York, 1978.
R. L. Heath,Int. Rev. Cytol.,34, 49 (1973).
A. J. Bearden and R. Malkin,Quart. Rev. Biophys.,7, 131 (1975).
L. L. Brown, R. M. Golding, P. C. Healy, K. J. Jessop and W. C. Tennant,Aust. J. Chem.,27, 2075 (1974).
W. Levason and C. A. McAuliffe,Coord. Chem. Rev.,7, 353 (1972).
M. Koikawa and H. Okawa,J. Chem. Soc., Dalton Trans., 641 (1988).
H. Okawa, M. Nakamura and S. Kida,Bull. Chem. Soc. Jpn.,55, 466 (1982).
S. Pal, G. Ghosh and A. Chakravorty,Inorg. Chem.,24, 3704 (1985).
S. E. Jones, D. Chin and D. T. Sawyer,Inorg. Chem.,20, 4257 (1981).
D. Chin, D. T. Sawyer, W. P. Schaefer and C. T. Simmons,Inorg. Chem.,22, 752 (1983).
D. T. Richens and D. T. Sawyer,J. Am. Chem. Soc.,101, 3681 (1979).
A. R. Hendrickson, R. L. Martin and N. M. Rohde,Inorg. Chem.,13, 1933 (1974).
R. Y. Saleh and D. K. Straub,Inorg. Chem.,13, 3017 (1974).
M. A. Suwyn and R. E. Hamm,Inorg. Chem.,6, 142, 2150 (1967).
T. E. Jones and R. E. Hamm,Inorg. Chem.,14, 1027 (1975).
D. J. Boone, R. E. Hamm and J. P. Hunt,Inorg. Chem.,11, 1060 (1972).
T. E. Jones and R. E. Hamm,Inorg. Chem.,13, 1940 (1974).
J. Stein, J. P. Fackler Jr., G. J. McClune, J. A. Fee and L. T. Chan,Inorg. Chem.,18, 3511 (1979).
P. Arselli and E. Mentasti,J. Chem. Soc., Dalton Trans., 689 (1983).
M. P. Heyward and C. F. Wells,J. Chem. Soc., Dalton Trans., 1331 (1988).
D. H. Macartney and D. W. Thompson,Inorg. Chem.,28, 2195 (1989).
R. E. Hamm and M. A. Suwyn,Inorg. Chem.,6, 139 (1967).
A. McAuley and U. D. Gomwalk,J. Chem. Soc., 977 (1969).
D. H. Macartney and A. McAuley,Inorg. Chem.,18, 2891 (1979) and references therein.
D. W. Carlyle and E. L. King,Inorg. Chem.,9, 2333 (1970).
R. G. Wilkins and R. E. Yelin,J. Am. Chem. Soc.,92, 1191 (1970).
R. W. Taft,J. Am. Chem. Soc.,75, 4231 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gangopadhyay, S., Saha, S.K. & Banerjee, P. Kinetics and mechanism of the reaction oftrans-cyclohexane-1,2-diamine-N N N' N'-tetra-acetatomanganate(III) with substituted thioureas in aqueous medium. Transition Met Chem 16, 355–357 (1991). https://doi.org/10.1007/BF01024082
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01024082