Abstract
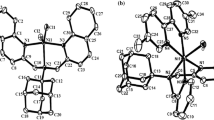
Copper(I) dimer [(DEED)CuBr]2 (4, DEED=N,N′-diethylethylenediamine) is rapidly oxidized by O2 to mixed valence peroxocopper complex [(DEED)CuBr]4O2 (1) in CH2Cl2 at −50 to 30°C. The long half- life for conversion of (1) into oxocopper(II) complex [(DEED)]CuBr]2O (3) allows (1), (3) and their carbonato derivative of [(DEED)CuBr]2CO3 (5) to be compared as oxidants of 2,6–dimethylphenol (DMPOH) to the corresponding diphenoquinone (DPQ) over a range of concentrations and temperatures. DPQ production is: 1)␣less than stoichiometric with deficits or slight excesses of DMPOH, but 2) mildly catalytic at moderate [DMPOH], as found with tetranuclear oxo-halo(pyridine)copper(II) oxidants. This behaviour is attributed to 1) co-product water destruction of initiators, and 2) inhibition by water of copper(I) reoxidation to complete the catalytic cycle. These inhibiting factors apparently are ameliorated by water incorporation in hydrogen-bonded phenol clusters in aprotic solvents. Initial rate measurements show that (1), (3) and (5) form monophenolate complexes with DMPOH in methylene chloride. The rate-determining step for conversion of these complexes to DPQ is fastest for oxocopper(II) complex (3) which is expected to be the strongest protic␣base. Highest rates with (3) and activation parameter comparisons suggest that the ability of phenolatocopper␣complexes to accept protons from coordinated phenolate is an important factor in determining overall copper- catalyzed phenolic oxidative coupling rates.
Similar content being viewed by others
References
A. S. Hay, Adv. Polym. Sci., 4, 496 (1967); A. S. Hay, P. Shenian, A. C. Gowan, P. F. Erhardt, W. R. Haaf and J. E. Therberg in Encyclopedia of Polymer Science and Technology, Interscience, New York, 1979, p. 92.; H. L. Finkbeiner, A. S. Hay and D. M. White in C. E. Schildnecht and I. Skeist (Eds.), Polymerization Processes, Wiley-Interscience, New York, 1977, p. 537 and refs therein.
Z. Tyeklar and K. D. Karlin, Accts. Chem. Res. 22, 241 (1989) and refs therein.
A. S. Hay, Polym. Eng. Sci., 16, 1 (1976).
A. Abu-Raqabah, G. Davies and M. A. El-Sayed, Inorg. Chim. Acta, 192, 31 (1992).
A. S. Hay, A. S. Blanchard, C. F. Endres and J. W. Eustance, J. Amer. Chem. Soc., 81, 6335 (1959).
G. Davies and M. A. El-Sayed in K. D. Karlin and J. Zubieta (Eds.), Copper Coordination Chemistry: Biochemical and Inorganic Perspectives, Adenine Press: Guilderland, New York, 1983, p. 281 and refs therein; G. Davies and M. A. El-Sayed, Inorg. Chem., 22, 1257 (1983).
I. Bodek and G. Davies, Inorg. Chem., 17, 1814 (1978).
G. Davies, M. A. El-Sayed and R. E. Fasano, Inorg. Chim. Acta, 71, 95 (1983).
G. Davies, M. A. El-Sayed, A. El-Toukhy, M. Henary and C. A. Martin, Inorg. Chem., 25, 4479 (1986).
M. A. El-Sayed, A. Abu-Raqabah, G. Davies and A. El-Toukhy, Inorg. Chem., 28, 1909 (1989).
D. A. Haitko and M. F. Garbauskas in K. D. Karlin and J. Zubieta (Eds.), Biological and Inorganic Copper Chemistry, Vol 2, Adenine Press, Guilderland, New York, 1986, p. 77.
M. A. El-Sayed, A. El-Toukhy and G. Davies, Inorg. Chem., 24, 3387 (1985).
G. Davies, M. F. El-Shazly and M. W. Rupich, Inorg. Chem., 20, 3757 (1981).
G. Davies, M. A. El-Sayed and M. Henary, Inorg. Chem., 26, 3266 (1987).
M. A. El-Sayed, T. A. El-Zayat and G. Davies, Inorg. Chim. Acta., 217, 194 (1994)
R. N. Keller, and H. D. Wycoff, Inorg. Synth., 2, 1 (1946).
M. A. El-Sayed and G. Davies, Inorg. Chem., 29, 4891 (1990).
R. M. Silverstein, G. C. Bassler and T. C. Morrell, Spectrometric Identification of Organic Compounds, 4th Edn., Wiley, New York, 1981, p 112.
G. Davies, Inorg. Chim. Acta, 160, 83 (1989).
G. Davies, X. Liu and M. A. El-Sayed, Inorg. Chim. Acta, 195, 35 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
El-Sayed, M.A., El-Wakil, H., Ismail, K.Z. et al. Stoichiometry, product and kinetics of catalytic oxidation of 2,6–dimethylphenol by bromo(N,N′- diethylethylenediamine)copper complexes in methylene chloride. Transition Metal Chemistry 23, 795–800 (1998). https://doi.org/10.1023/A:1006970209841
Issue Date:
DOI: https://doi.org/10.1023/A:1006970209841